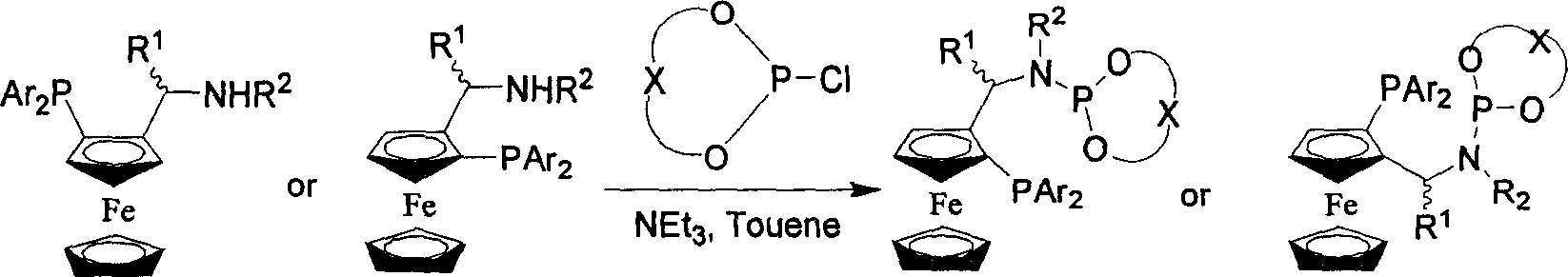
Catalyst using phosphine-phosphoramidite ester as ligand, its preparation method and application
A phosphoramidite and catalyst technology, applied in the direction of organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problem of poor activity and enantioselectivity question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Add 7.2 grams of (R)-1-(dimethylamino)ethylferrocene and 40ml of ether to a 250ml three-necked flask, and slowly add 16.1ml of n-BuLi hexane solution with a concentration of 2.15mol / l at room temperature . After the addition was complete, the stirring reaction was continued at room temperature for 1.5 hours. The reaction mixture was warmed up to slightly boiling state, slowly added 12.4 grams of ClPPh 2 (diphenylphosphine chloride) and 20ml of diethyl ether to form a solution. After the addition was complete, the reaction was continued to reflux and stirred for 4 hours. Cool the reaction night to 0 °C, slowly add 40 ml saturated NaHCO 3(Sodium bicarbonate) aqueous solution, the addition was completed, and the stirring reaction was continued for 10 minutes. Separation, the aqueous layer was extracted twice with 50ml ether, combined ether layer, washed with 50ml water, anhydrous Na 2 SO 4 dry. Filtrate, remove the solvent under reduced pressure, and recrystallize t...
Embodiment 2
[0082] Dissolve 10.0g of (S)-1-(dimethylamino)ethylferrocene in 60ml of anhydrous ether, add 21.8ml of n-BuLi hexane solution with a concentration of 2.05mol / l at room temperature, and add for about 30 minutes. Finish. After continuing to stir and react at room temperature for 1 hour, the temperature was raised to reflux, and a solution formed by adding 6.4 g of trimethylchlorosilane and 25 ml of ether was added within 1.5 hours. The reaction solution was cooled to 5°C, and 50ml of water was added. Separate the organic phase and wash with 8.5% HO 3 PO 4 Aqueous extraction. Combine the aqueous phases with 10% Na 2 CO 3 Aqueous solution neutralized to slightly alkaline, CHCl 3 Extraction, anhydrous Na 2 SO 4 dry. The solvent was removed under reduced pressure, and the residue was purified by column chromatography (silica gel, triethylamine pretreatment, petroleum ether / ethyl acetate / triethylamine: 30 / 1 / 0.5). The solvent was removed to obtain 7.8 g of (S, R)-2[(S)-N,N-d...
Embodiment 3
[0089] Under nitrogen protection, 2.0mg (0.005mmol) [Rh(COD) 2 ] BF 4 (bicyclooctadiene rhodium complex tetrafluoroborate), the above preparation (R, S, R)-DicpPhos (0.011mmol) and solvent dichloromethane (1.5ml) are placed in a 10ml reactor, reacted for 30 After 1 min, the substrate methyl 2-acetamidoacrylate (0.5 mmol) was added with 1.5 ml CH 2 Cl 2 The formed solution was replaced with hydrogen for 3 times, and the reaction was terminated after maintaining normal pressure for 30 minutes, filtered with a short silica gel column, and after the filtered filtrate was concentrated, the content and optical purity were determined by GC to obtain S-acetylaminopropionic acid formazan The ester yield is 100% (calculated as 2-acetamidomethyl acrylate), and the enantiomeric excess is above 99% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com