Process for preparing anatase type nano crystal TiO2 solar energy cell material
A solar cell and anatase-type technology, applied in circuits, electrical components, titanium dioxide, etc., can solve the problems of slow electron transfer, affecting the photoelectric conversion efficiency of dye-sensitized solar cells, and affecting the photoelectric performance of solar cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
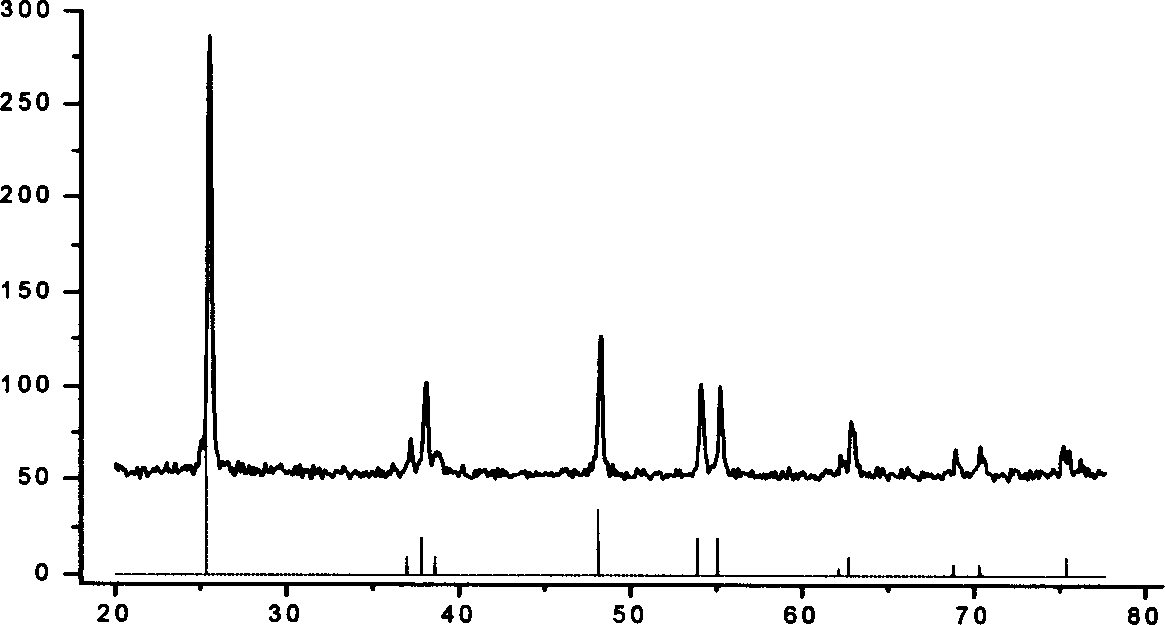
[0020] Isopropyl titanate was added dropwise to deionized water cooled to 0° C. with ice water to obtain a white precipitate. After separation and washing, the precipitate is transferred to tetramethylammonium hydroxide solution, wherein the concentration of tetramethylammonium hydroxide is controlled by the molar ratio of isopropyl titanate and tetramethylammonium hydroxide at 62.5:1, under stirring Reflux at 100°C to obtain a colloidal solution. The above colloid was transferred to a high-pressure tank lined with polytetrafluoroethylene, kept at 210°C for 12 hours, centrifuged to remove alkali, and dried to obtain anatase titanium dioxide nanocrystals with a particle size of 14nm.
Embodiment 2
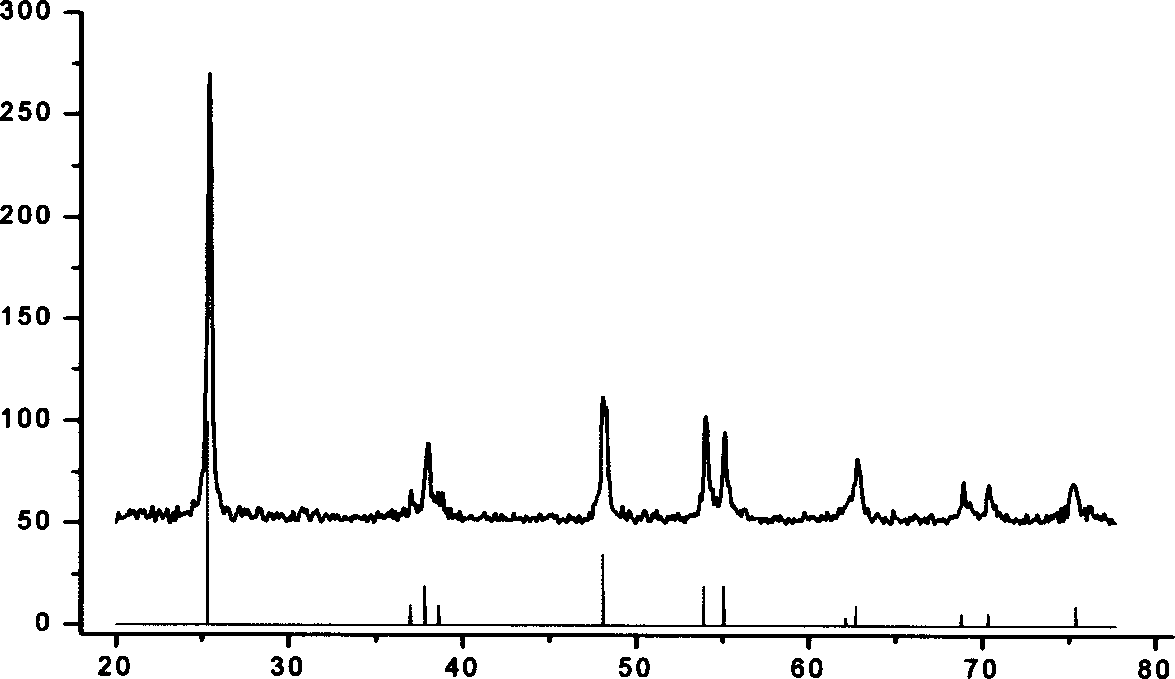
[0022] Tetrabutyl titanate was added dropwise into deionized water at 20° C. to obtain a white precipitate. After separation and washing, the precipitate is transferred to the solution of tetraethylammonium hydroxide, wherein the concentration of tetraethylammonium hydroxide is controlled by the molar ratio of tetrabutyl titanate to tetramethylammonium hydroxide as 62.5:4, and the Reflux at 120°C to obtain a colloidal solution. The above colloid was transferred to a high-pressure tank lined with polytetrafluoroethylene, kept at 230°C for 24 hours, centrifuged to remove alkali, and dried to obtain anatase titanium dioxide nanocrystals with a particle size of 30nm.
Embodiment 3
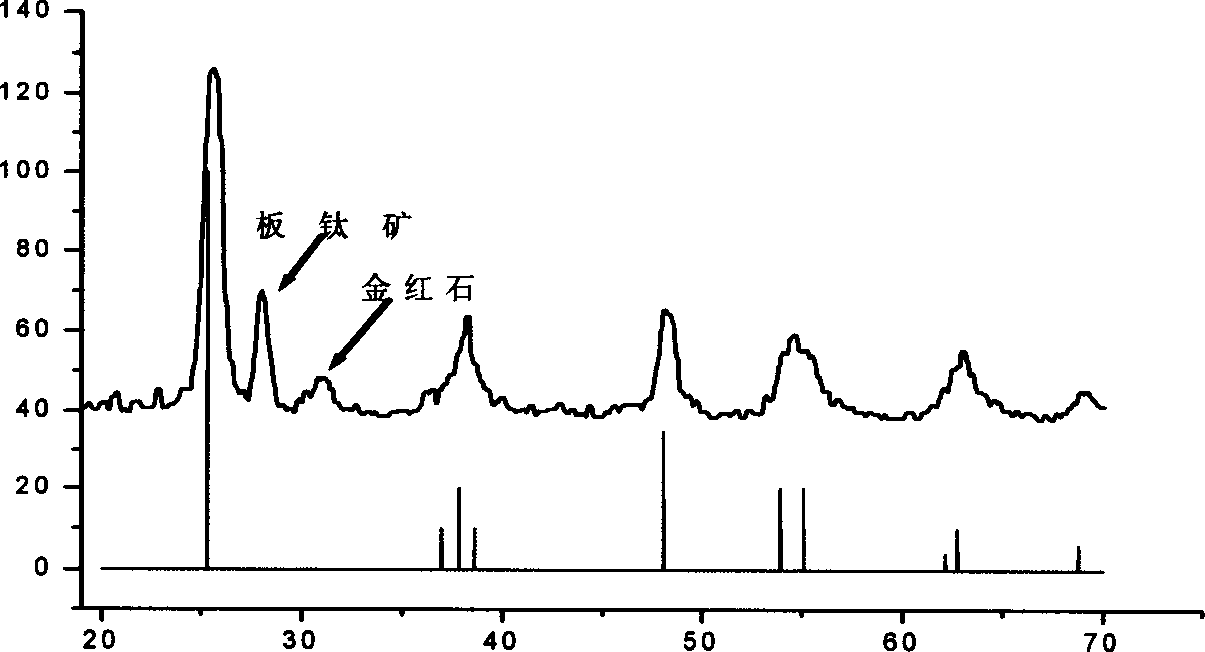
[0024] Isopropyl titanate was added dropwise to deionized water at 15 °C to obtain a white precipitate. After separation and washing, the precipitate is transferred to the solution of tetramethylammonium hydroxide, wherein the concentration of tetramethylammonium hydroxide is controlled by the molar ratio of isopropyl titanate and tetramethylammonium hydroxide at 750:1, and the Reflux at 100°C to obtain a colloidal solution. The above colloid was transferred to a high-pressure tank lined with polytetrafluoroethylene, kept at 190° C. for 4 hours, centrifuged to remove alkali, and dried to obtain anatase titanium dioxide nanocrystals with a particle size of 8 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap