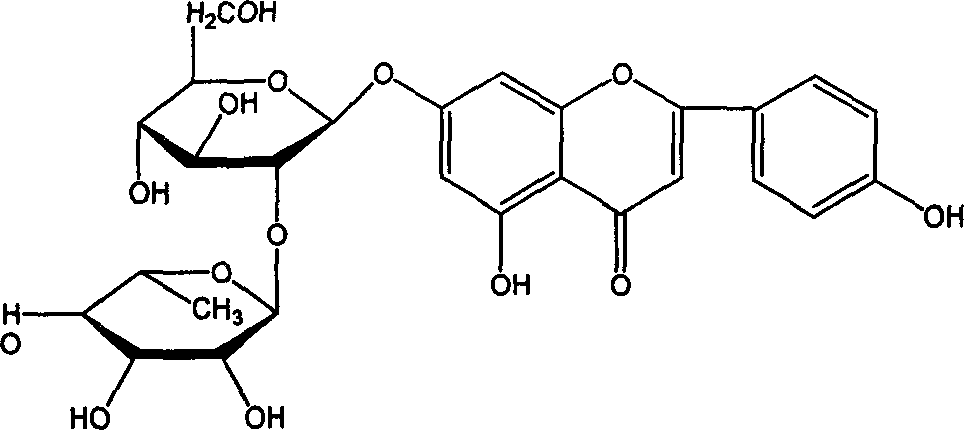
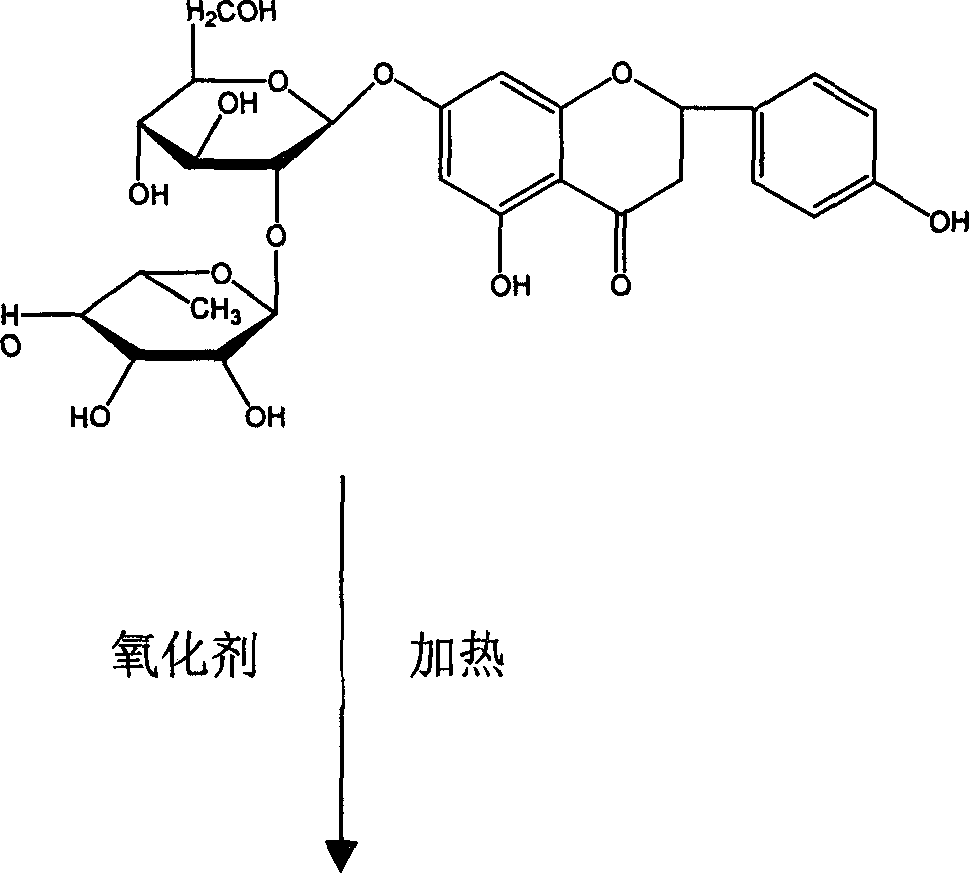
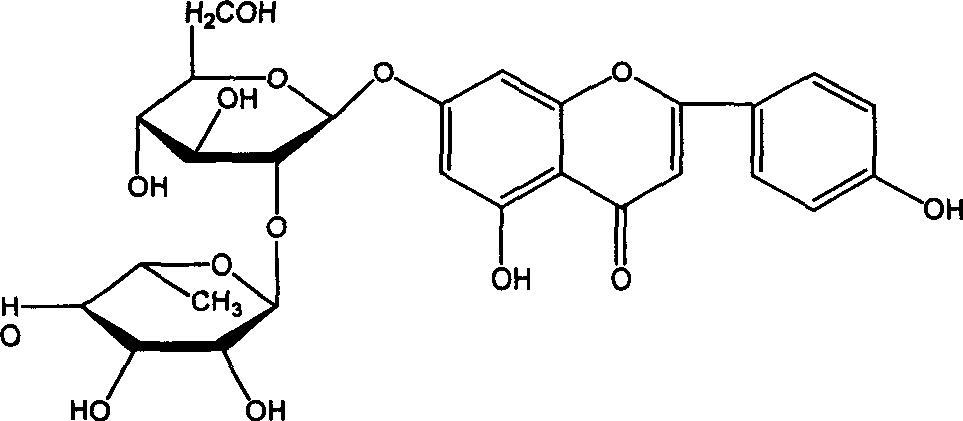
Process for synthesizing lacquer leafide
A synthesis method and technology of aruginin, which is applied in the field of synthesis of aruticin, can solve the problems of difficult industrial production, unfriendly smell of human body, increased production cost, etc., and achieves low price, easy control of reaction conditions, and reduced synthesis cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Take 70g of naringin, dissolve it in 350ml of 1,4-dioxane, add 2.5g of sodium hydroxide at the same time, put it in a three-necked flask, and keep it under reflux; take 24.4g of iodine and dissolve it in 150ml of 1,4-dioxane Pour the oxycyclohexane into a constant-pressure dropping funnel, slowly drop it into the above-mentioned three-necked flask, and perform an oxidation reaction at 105° C. for 4 hours. After the reaction, concentrate the reaction solution to 100ml, add water to 1000ml, let stand for 12 hours, and filter. Dissolve with 200ml of 7% aqueous sodium hydroxide solution, then add 200ml of ethanol, and adjust the pH to 4 with hydrochloric acid. Let stand to precipitate and filter. 100ml of 1,4-dioxane was used to heat and dissolve the arucidin obtained above, then water was added, and 42g of dry cake was obtained by filtration. Repeat the recrystallization with 1,4-dioxane / water twice, and filter and dry to obtain 38 g of aucidin with a purity higher than ...
Embodiment 2
[0020] Take 70g of naringin and 30.5g of iodine and dissolve them in 400ml of 1,4-dioxane, add 3.2g of potassium hydroxide, keep the constant temperature water bath at 95°C, and conduct the oxidation reaction for 7h. After the reaction was completed, the reaction liquid was concentrated and added with water as in Example 1, left to stand overnight, and filtered to obtain the crude product aruginin. Then add 200ml of 3% sodium hydroxide aqueous solution to dissolve, then add 200ml of methanol, and adjust the pH to 6 with sulfuric acid. Let stand to precipitate and filter. Heat 1,4-dioxane to dissolve the arucidin obtained above, then add water, filter and dry to obtain 46 g of aruginin with a purity higher than 98%, and the yield is 65.7%.
Embodiment 3
[0022] Take 70g of naringin, dissolve it in 250ml of 1,4-dioxane, add 3.0g of sodium hydroxide and place it in a 1000ml three-necked flask, and keep the water bath at a constant temperature of 60°C. Dissolve 30.5g of iodine in 150ml of 1,4-dioxane, first add 50ml of iodine in 1,4-dioxane to the there-necked bottle, and the remaining iodine in 1,4-dioxane The alkane solution was added into the three-necked flask three times, about once every 1 hour. After the reaction, the reaction solution was concentrated and added with water according to Example 1, left to stand overnight, and filtered to obtain a crude product. Heat 100ml of dimethylformamide to dissolve the arugenin obtained above, filter, then add water to 500ml, let stand and filter. Then add 500 ml of 5% sodium hydroxide aqueous solution to dissolve, then add 500 ml of glycerin, and adjust pH=5 with sulfuric acid. Let stand to precipitate and filter. Obtained 26g of aruticin with a purity higher than 98%, and the yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com