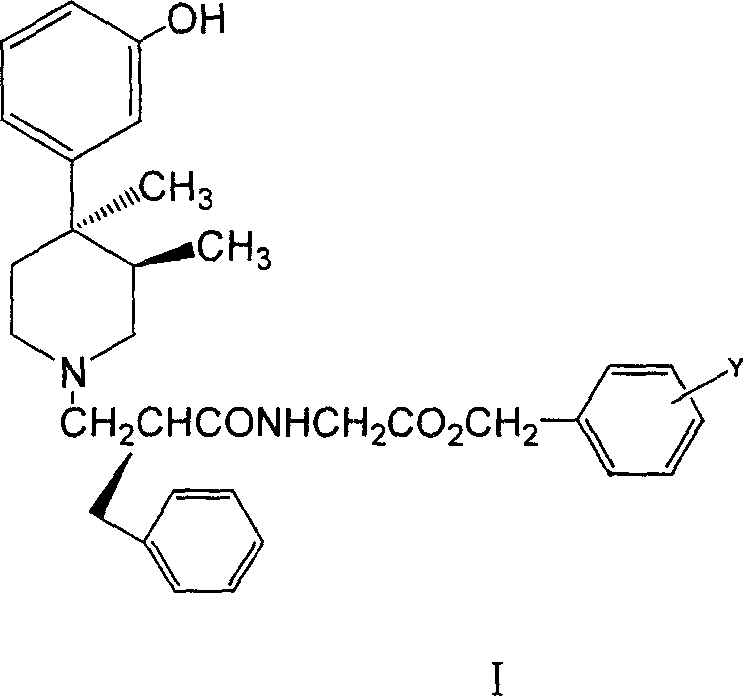
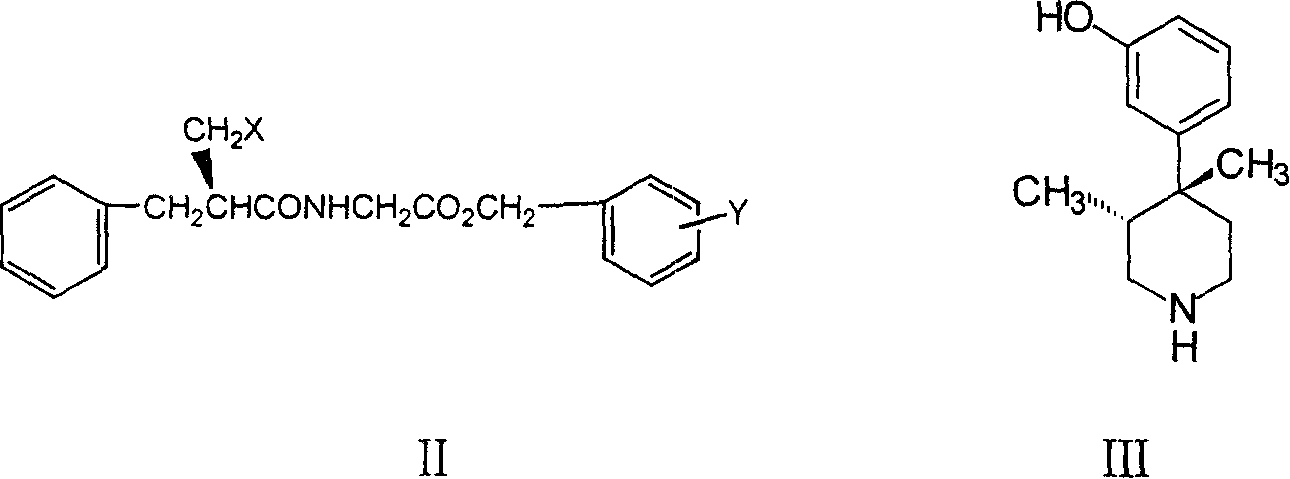
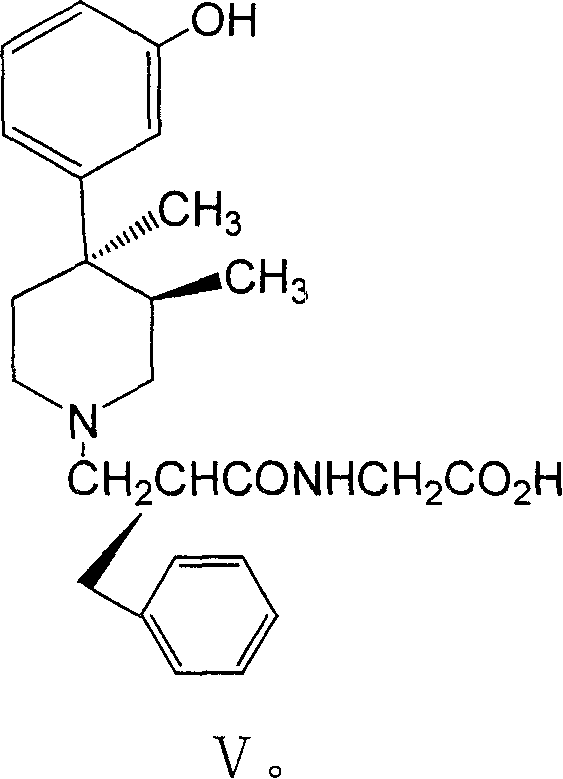
Substituted benzyl ester and its preparation process and novel process for preparing substituted mopipe therefrom
A compound and chemical technology, applied in the field of pharmaceutical chemical synthesis, can solve the problems of inconvenient large-scale production and harsh storage conditions, and achieve the effects of shortening synthesis steps, reducing synthesis costs and mild synthesis reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0032] 1. Preparation example 1: Preparation of (S)-2-halomethylphenylpropionyl-glycine benzyl ester
[0033] 1, Preparation of 3-hydroxyl-2-methenyl-3-phenylpropionic acid methyl ester
[0034] Add 67.3g of benzaldehyde, 60ml of methyl acrylate, and 13.5g of triethylenediamine into a 500ml reaction bottle, stir at room temperature for 5 days, add 60ml of water, 60ml of concentrated hydrochloric acid, and 120ml of ethyl acetate. layer, the organic layer was washed twice with 20ml of water, the organic layer was dried with anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 110.5 g of light yellow oil, which was distilled under reduced pressure to collect fractions at 85-90°C / 7-9mmHg. 97.5 g of colorless liquid was obtained, the content was greater than 98% (GC method), and the yield was 80%.
[0035] 2, Preparation of 2-hydroxymethylbenzeneacrylic acid
[0036] Dissolve 60g of 3-hydroxy-2-methenyl-3-phenylpropionic acid m...
preparation example 2
[0053] Two, Preparation Example 2: Preparation of (+)-3R, 4R-(3-hydroxyphenyl)-3,4-dimethylpiperidine
[0054] 1, the preparation of 1,3-dimethyl-4-(3-isopropoxyphenyl)-4-hydroxypiperidine
[0055] Under the condition of nitrogen protection, 60g of 3-bromocymene was added to 150ml of THF, cooled to -72°C, and 150ml of n-butyllithium (2.5M) was slowly added dropwise. After dropping, stir at -60--70°C for 1 hour. Then 30 g of 1,3-dimethyl-4-piperidone was added dropwise, the reaction temperature was kept at about -60--70°C, and stirred for 1 hour. Add 6N hydrochloric acid to the reaction solution under stirring, adjust the pH to 1-2, separate the water layer, adjust the pH to 12 with 20% sodium hydroxide, extract with ethyl acetate three times, combine the organic layers, and dry over anhydrous sodium sulfate overnight. After filtration, the filtrate was concentrated to dryness under reduced pressure, and recrystallized from n-heptane. After filtering, the product was dried ...
Embodiment 1
[0070] 1. (+)-(3R,4R)-[[2S-[[4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl]-1-oxo - Preparation of 3-phenylpropyl]amino]acetic acid-4'-bromobenzyl ester
[0071] Method A:
[0072] 10g (+)-3R, 4R-(3-hydroxyphenyl)-3,4-dimethylpiperidine, 25.9g (S)-2-methanesulfonylmethylbenzoyl-4'-bromo -benzyl glycine, the Virahol of 300ml, the potassium carbonate of 9.6g, the potassium iodide of 0.1g was added in the three-necked reaction flask of 500ml, heated and refluxed for 8 hours, and the TCL method detected that the basic reaction of the reaction raw materials was complete (R f value is about 0.4, ethyl acetate / petroleum ether=1 / 15), cooled to room temperature, filtered, and concentrated under reduced pressure to obtain about 28.2 g of yellow oil. The content of the prepared product is about 80.4% (HPLC method), and the yield is 78.6%. It can be directly put into the next step for reaction.
[0073] Take 1 gram of oily matter and pass through column separation and purifica...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com