Method for quick screening angiotemsin invertase inhibitor combined using high effieient liquid chromatograph and mass spectrum
A high-performance liquid chromatography, angiotensin technology, applied in the field of analytical chemistry, can solve problems such as long time, achieve the effect of simple operation, accurate and reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Preparation solution: buffer solution A: buffer solution (pH=8.3) of 300 mmol / liter sodium chloride and 100 mmol / liter tris and formic acid;
[0036] Buffer solution B: a buffer solution of 300 mmol / L sodium chloride and 100 mmol / L Tris and formic acid (pH=8.0).
[0037] Use buffer solution B to prepare a 4-8 mmol / L hippuric acid diglyglyptide solution, and use buffer A to prepare a 4-8 mmol / L hippuric acid histidine-leucine solution.
[0038] Prepare purified rabbit pulmonary angiotensin-converting enzyme solutions with buffer solutions A and B, respectively.
[0039] (2) Experiment:
[0040] 2 mmol / L Glypeptide hippurate substrate reacted with blank plasma, the total reaction volume was 100 μl
[0041] Control group: 40 microliters of 4 mmol / L diglyglycerin hippurate+20 microliters (pH=8.0) of buffer solution B+40 microliters of blank plasma
[0042] Test group: 40 microliters of 4 mmol / L hippuric acid diglycyl peptide + 20 microliters of 0.8 ng / ml benazeprilat...
Embodiment 2
[0081] 2 mmol / L hippurate substrate and purified rabbit pulmonary angiotensin-converting enzyme (2 mU) were reacted in a total reaction volume of 80 μl
[0082] Control group: 40 microliters of 4 mmol / L hippuric acid diglycyl peptide + 20 microliters of buffer solution B + 20 microliters of angiotensin converting enzyme (2 milliU)
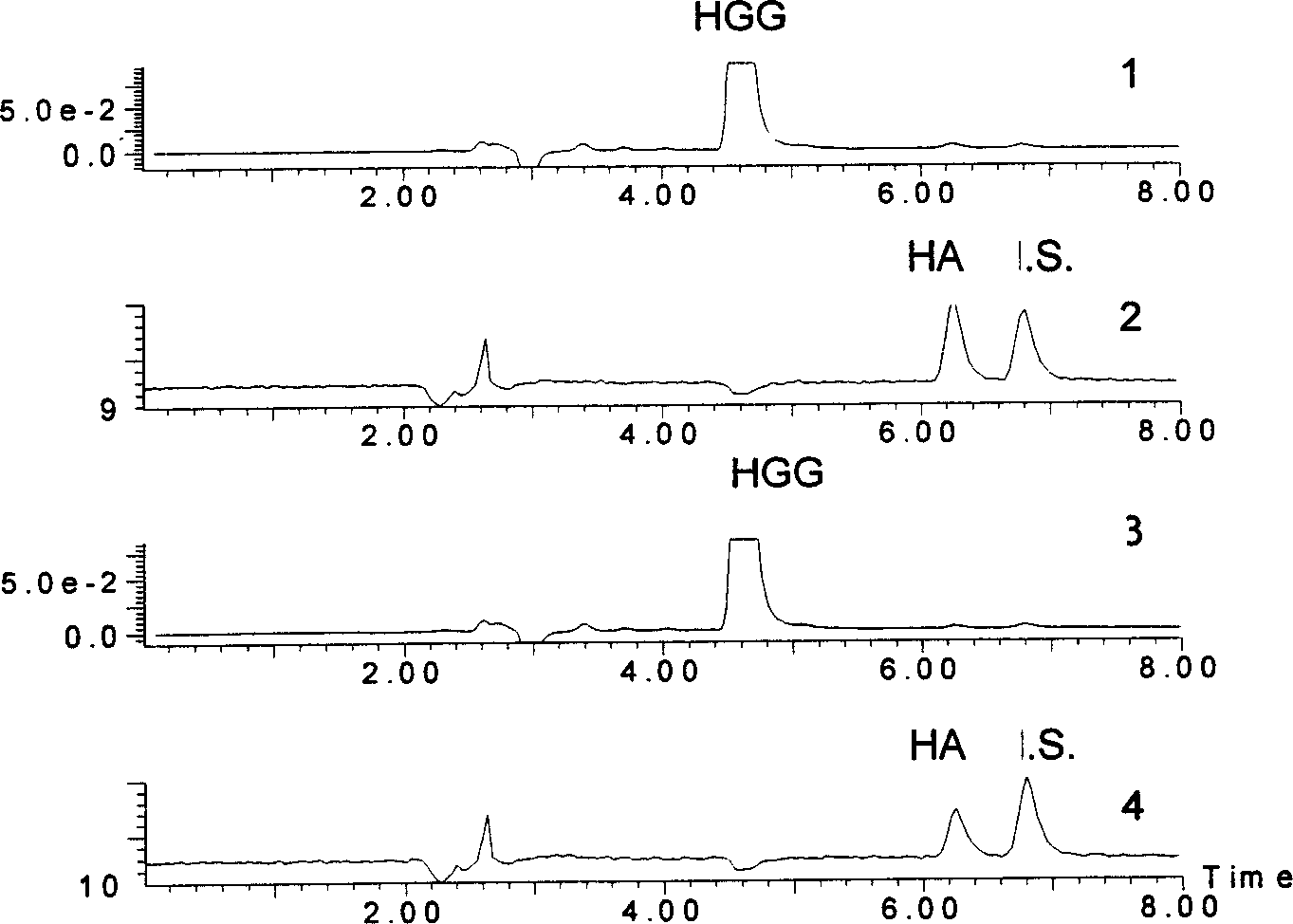
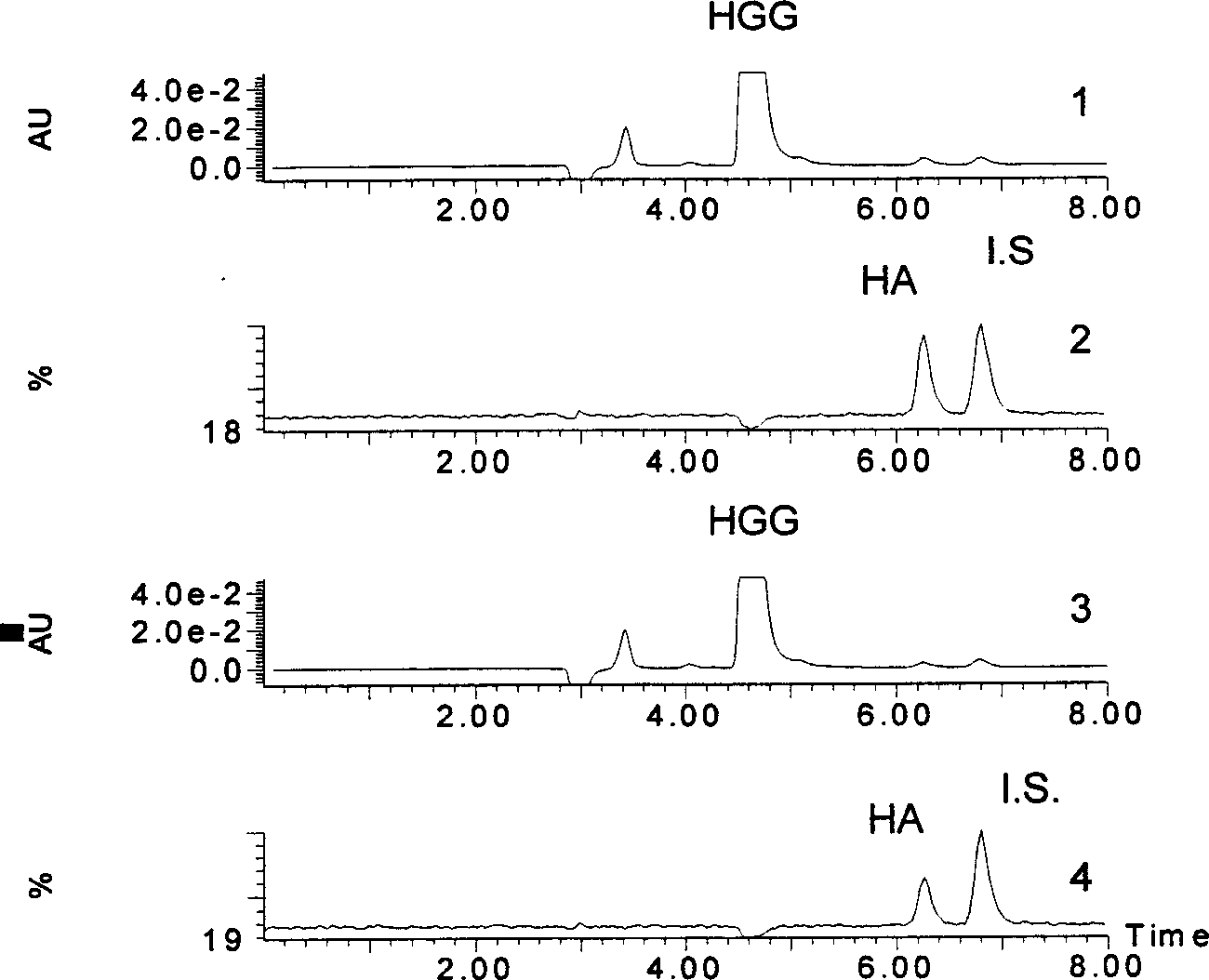
[0083] Experimental group: 40 microliters of 4 mmol / L hippuric acid diglycyl peptide + 20 microliters of 0.8 ng / ml benazeprilat solution + 20 microliters of angiotensin-converting enzyme (2 milliliters U) in a constant temperature water bath at 37°C After reacting in the pot for 8 minutes, add 160 microliters of acetonitrile solution containing internal standard terephthalic acid, vortex and shake for a few seconds, filter with a 0.45 micron water membrane, and analyze according to the steps of Example 1. The result is as figure 2 shown.
Embodiment 3
[0085] 2 mmol / L hippurate histidine leucine substrate reacted with blank plasma, total volume 100 µl
[0086] Control group: 40 μl 4 mmol / L hippuric acid histidine leucine + 20 μl buffer solution B + 40 μl blank plasma
[0087] Test group: 40 μl 4 mmol / L hippurate histidine leucine + 20 μl 0.8 ng / ml Benazeprilat solution + 40 μl blank plasma
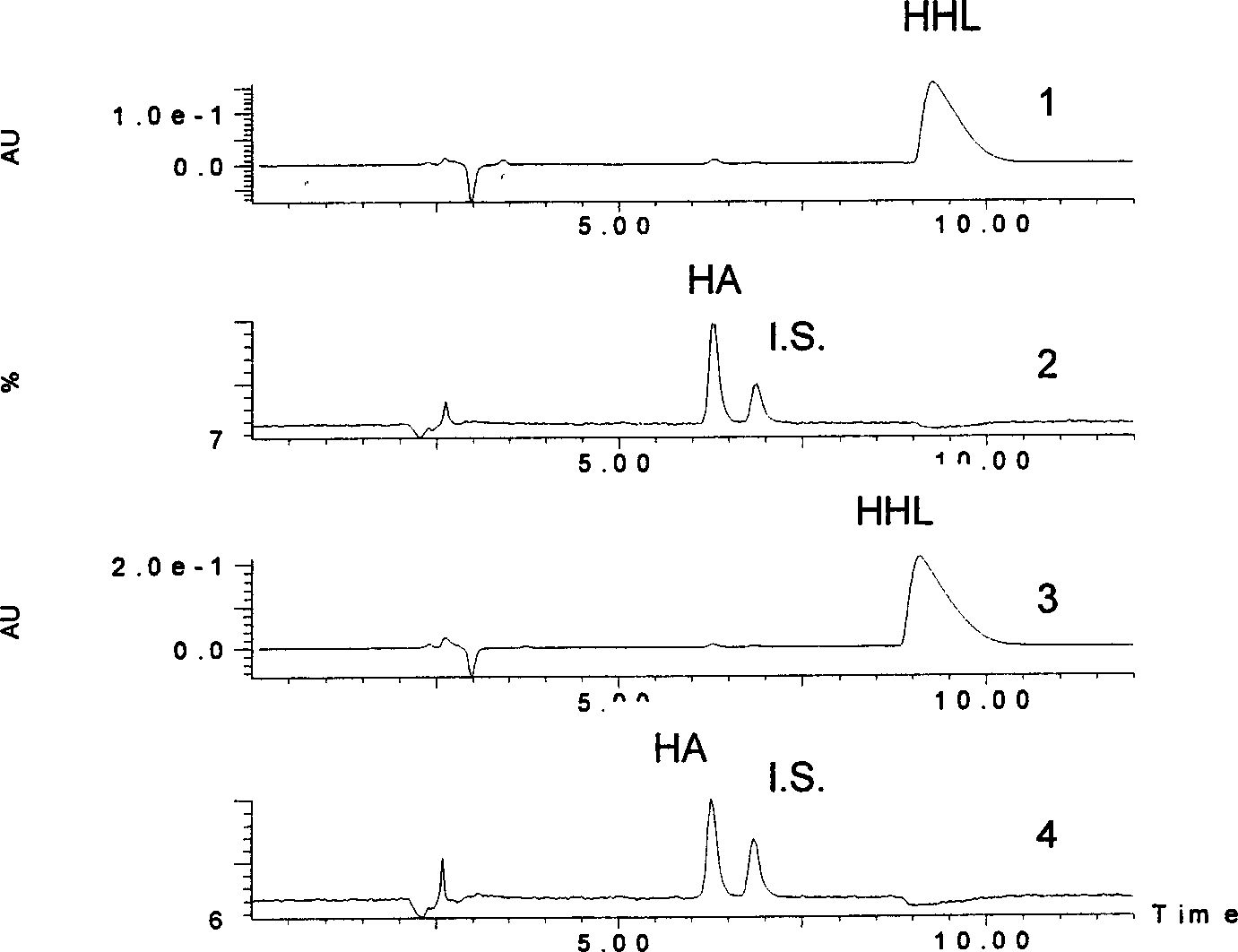
[0088] After reacting in a constant temperature water bath at 37° C. for 16 minutes, add 200 microliters of acetonitrile plus internal standard terephthalic acid, centrifuge at 10,000 rpm for 8 minutes, and analyze the supernatant according to the steps in Example 1. The result is as image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com