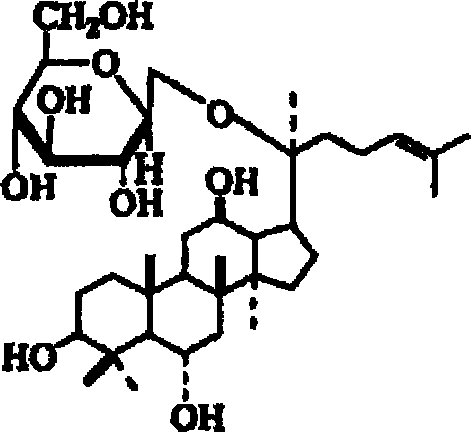
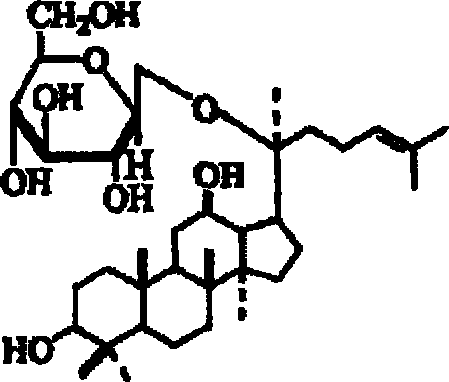
A composition containing ginsenoside F1 or compound K for skin external application
A technology of ginsenosides and compositions, applied in the field of external skin compositions containing ginsenoside F1 or compound K
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0033] Reference Example 1 Purification of Ginsenosides
[0034] 2 kg of red ginseng (KT&G company, 6-year-old red ginseng) was added to 4 liters of aqueous ethanol, refluxed at 77°C for 3 times, and then precipitated at 15°C for 6 days. Residues and filtrates were separated by filtration and centrifugation, and then the filtrate was concentrated under reduced pressure to obtain an extract. The extract was suspended in water, extracted 5 times with 1 liter of ether to remove the pigment, and the resulting aqueous layer was extracted 3 times with 500 ml of 1-butanol. The obtained 1-butanol layer was treated with 5% KOH and washed with distilled water, and concentrated under reduced pressure to obtain a 1-butanol extract. The 1-butanol extract was dissolved in a small amount of methanol, and a large amount of ethyl acetate was added thereto to obtain a precipitate. The precipitate was dried to obtain 100 g of purified ginsenoside (yield: 5%). The same operation as above was r...
Embodiment 1
[0036] Example 1 Preparation of Ginsenoside F1 by Acid Hydrolysis
[0037] The purified ginsenoside obtained in 100 grams of reference example 1 was dissolved in 20 times the amount (volume / weight) of sulfuric acid / 50% ethanol solution (volume / weight), and refluxed in a water bath at 100° C. for 6 hours to hydrolyze saponins Glycoside sugar bond. The reactant was concentrated under reduced pressure to remove all solvent, and the residue was suspended in 1000 mL of distilled water. Then it was extracted three times with diethyl ether, each time with the same amount of diethyl ether. The entire ether layer was washed with distilled water, then dried over anhydrous magnesium sulfate, filtered and concentrated to give crude product. The crude product obtained was chromatographed on silica gel (separation was carried out by increasing the polarity by changing the ratio of chloroform to methanol from 9:1 to 4:1). Each aliquot was passed through thin film chromatography (chlorofor...
Embodiment 2
[0038] Example 2 Preparation of Compound K by Enzymatic Hydrolysis
[0039] 100 g of the purified ginsenoside obtained in Reference Example 1 was dissolved in citrate buffer (pH 5.5). 1 g of naringinase isolated from Penicillium sp. was added thereto, and the mixture was reacted in a water bath at 40° C. under stirring for 48 hours. Periodic monitoring was performed using thin film chromatography, and when the substrate was completely removed, the reaction was terminated by heating in a hot water bath for 10 min. The reactant was then extracted three times with the same amount of ether and concentrated. The resulting product was chromatographed on silica gel (separation was achieved by increasing the polarity by changing the ratio of chloroform to methanol from 9:1 to 4:1). Each aliquot was passed through thin film chromatography (chloroform / methanol / water=65 / 35 / 10, R f =0.73), to isolate an aliquot of Compound K, finally obtaining 4400 mg of Compound K (yield: 4.4%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com