Phosphine-based Marpropy multipolymer containing fluorescent base-group and its production
A technology of horse-propylene copolymer and fluorescent group, which is applied in the field of phosphino-mercapto-propylene copolymer and its preparation, can solve the problems of limited development and application, rare and difficult reaction raw materials, etc., to reduce interference, simplify on-site operation, improve The effect of management level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
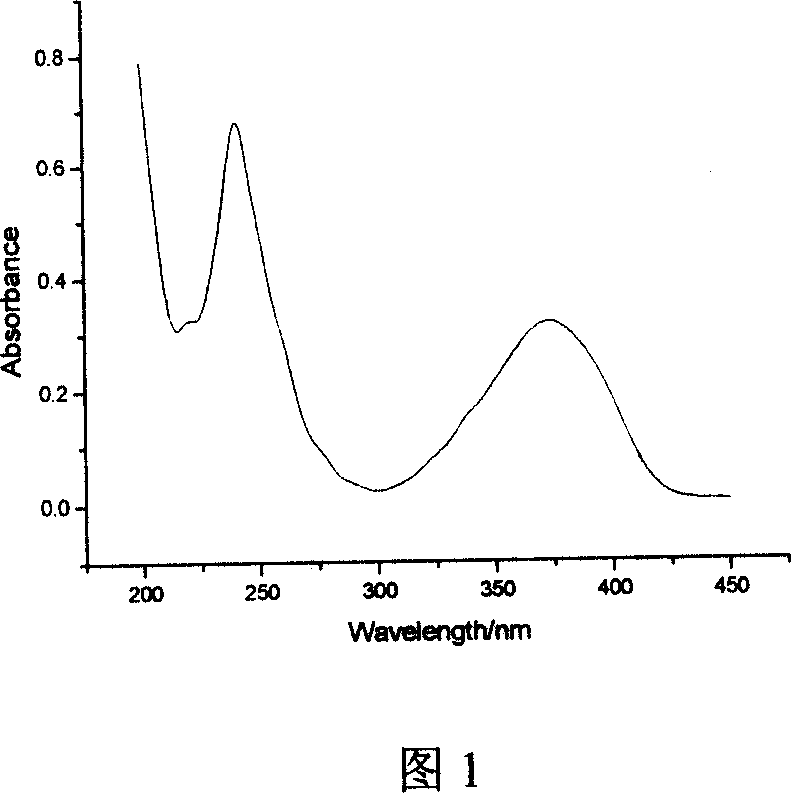
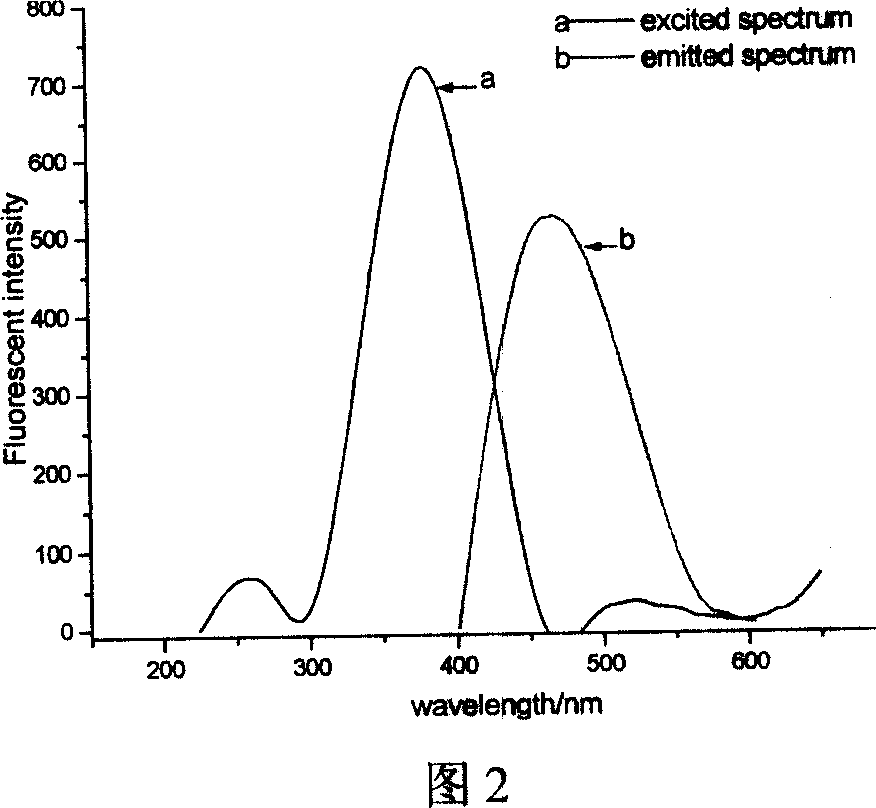
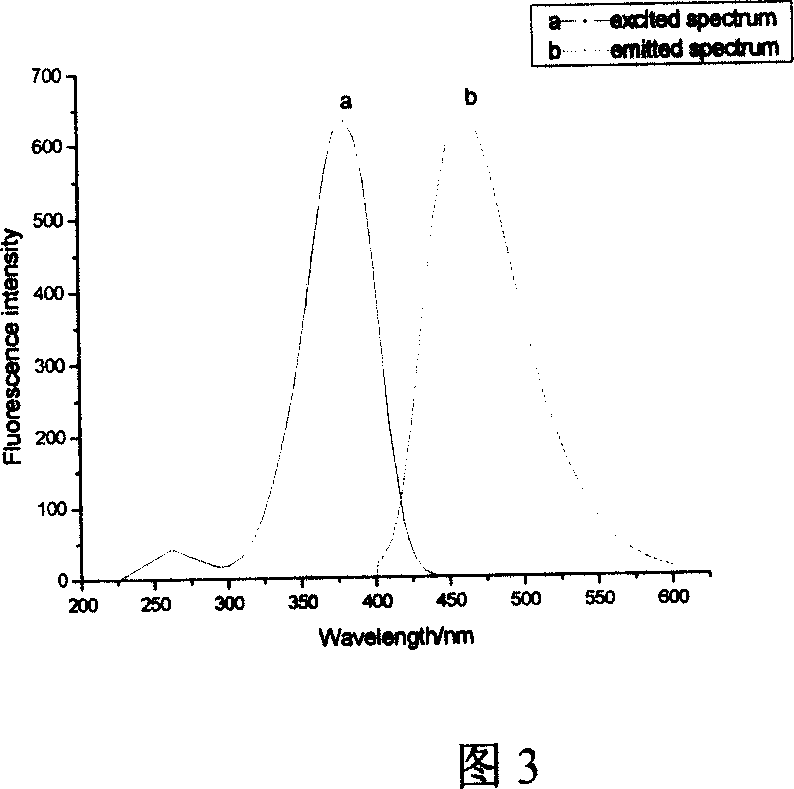
[0034] Embodiment 1: In conjunction with Fig. 1 to Fig. 5, add 5.6g (being 0.02mol) 4-bromo-1,8-naphthalene dicarboxylic anhydride, 50mL glacial acetic acid in the three-necked flask, mechanically stir, dropping funnel drips N , N-dimethylethylenediamine 2.6mL (ie 0.024mol), under nitrogen protection, heated to reflux for 5h. After the reaction, 30 mL of deionized water was added, and neutralized with 50% (mass fraction) sodium hydroxide until the pH was 8-9. Suction filtration, the solid was washed with water to obtain a khaki solid. Recrystallized with DMF to obtain 5.23 g of compound 4-bromo-N-(2-N',N'-dimethylaminoethyl)naphthalimide, with a yield of 75.2%.
[0035] Weigh 4.1 g (ie 0.012 mol) of the compound 4-bromo-N-(2-N', N'-dimethylaminoethyl) naphthalimide into a three-necked flask, add 50 mL of methanol, 1.3 g (that is, 0.024mol) sodium methoxide, heated in a water bath, protected by nitrogen, stirred mechanically, and reacted for 6 hours. After the reaction, neut...
Embodiment 2
[0040] Embodiment 2: In conjunction with Fig. 1 to Fig. 5, add 10.08g (being 0.036mol) 4-bromo-1,8-naphthalene dicarboxylic anhydride, 80mL glacial acetic acid in the three-necked flask, mechanically stir, dropping funnel drips N , N-dimethylethylenediamine 3.3mL (ie 0.03mol), under nitrogen protection, heated to reflux for 5h. After the reaction, 50 mL of deionized water was added, and neutralized with 50% (mass fraction) sodium hydroxide until the pH was 8-9. Suction filtration, the solid was washed with water to obtain a khaki solid. Recrystallized with DMF to obtain 6.94 g of light yellow product 4-bromo-N-(2-N',N'-dimethylaminoethyl)naphthalimide, with a yield of 66.5%.
[0041] Weigh 4.1 g (0.012 mol) of the compound 4-bromo-N-(2-N', N'-dimethylaminoethyl) naphthalimide into a three-necked flask, add 50 mL of methanol, 0.975 g (that is, 0.018mol) sodium methoxide, heated in a water bath, protected by nitrogen, stirred mechanically, and reacted for 6 hours. After the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
| scale inhibition rate | aaaaa | aaaaa |
| scale inhibition rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com