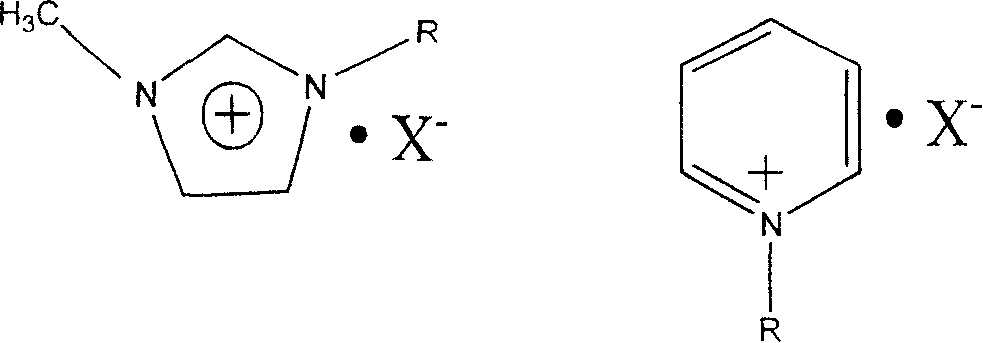Microwave synthesis for producing polyketone ionic liquid
A technology for ionic liquid and microwave synthesis, which is applied in the field of microwave synthesis to achieve the effects of simple operation, environmental friendliness and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 0.5mol N-methylimidazole and 0.6mol n-butane bromide successively into two-necked flasks (special microwave instruments), and carry out ionization in the WF-4000 atmospheric pressure microwave rapid reaction system purchased by Shanghai Yiyao Analytical Instrument Co., Ltd. Liquid preparation. Magnetic stirring, microwave power of 300W, 80°C, 4min to end the reaction. The product was washed three to four times with ethyl acetate, and dried under vacuum at room temperature for 6 h to obtain 107.3 g of 1-n-butyl-3-methylimidazolium bromide with a yield of 98%.
[0017] Add 0.5 mol of 1-n-butyl-3-methylimidazolium bromide, 0.6 mol of sodium fluoroborate, and 150 ml of deionized water into a two-neck bottle (special microwave instrument), stir magnetically, and set the microwave power to 300W. 100°C, 10 minutes to complete the reaction, a clear and transparent light yellow liquid can be obtained. After the liquid is cooled, add the prepared lead fluoroborate aqueous s...
Embodiment 2
[0022]Add 0.5 mol N-methylimidazole and 0.6 mol bromo-n-butane into a two-neck bottle (special microwave equipment) in sequence, stir magnetically, microwave power is 200W, 80°C, 10min to end the reaction. The product was washed three to four times with ethyl acetate, and dried under vacuum at room temperature for 6 h to obtain 106.2 g of 1-n-butyl-3-methylimidazolium bromide with a yield of 97%.
[0023] Add 0.5 mol of 1-n-butyl-3-methylimidazolium bromide, 0.6 mol of sodium fluoroborate, and 150 ml of deionized water into a two-neck bottle (special microwave instrument), stir magnetically, and set the microwave power to 200W. 100°C, 15 minutes to complete the reaction, a clear and transparent light yellow liquid can be obtained. After the liquid is cooled, add the prepared lead fluoroborate aqueous solution to it dropwise until no white precipitate is formed in the solution. At 0°C, let it stand for more than 6 hours. After filtering and collecting the filtrate, the water ...
Embodiment 3
[0025] Add 0.5 mol N-methylimidazole and 0.6 mol bromo-n-butane into a two-neck flask (special microwave equipment) in sequence, stir magnetically, microwave power is 400W, 80°C, 3min to end the reaction. The product was washed three to four times with ethyl acetate, and dried under vacuum at room temperature for 6 h to obtain 105.1 g of 1-n-butyl-3-methylimidazolium bromide with a yield of 96%.
[0026] Add 0.5 mol of 1-n-butyl-3-methylimidazolium bromide, 0.6 mol of sodium fluoroborate, and 150 ml of deionized water into a two-necked bottle (special equipment for microwave), stir magnetically, and set the microwave power to 400W. 100°C, 8min to end the reaction. , a clear and transparent light yellow liquid can be obtained. After the liquid is cooled, add the prepared lead fluoroborate aqueous solution to it dropwise until no white precipitate is formed in the solution. At 0°C, let it stand for more than 6 hours. After filtering and collecting the filtrate, the water was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com