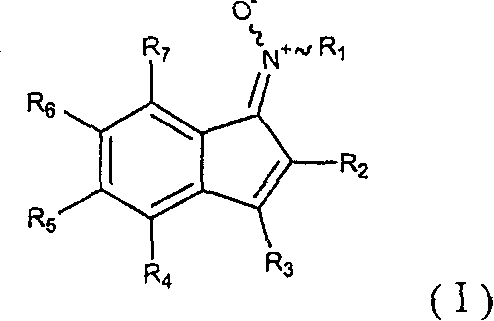
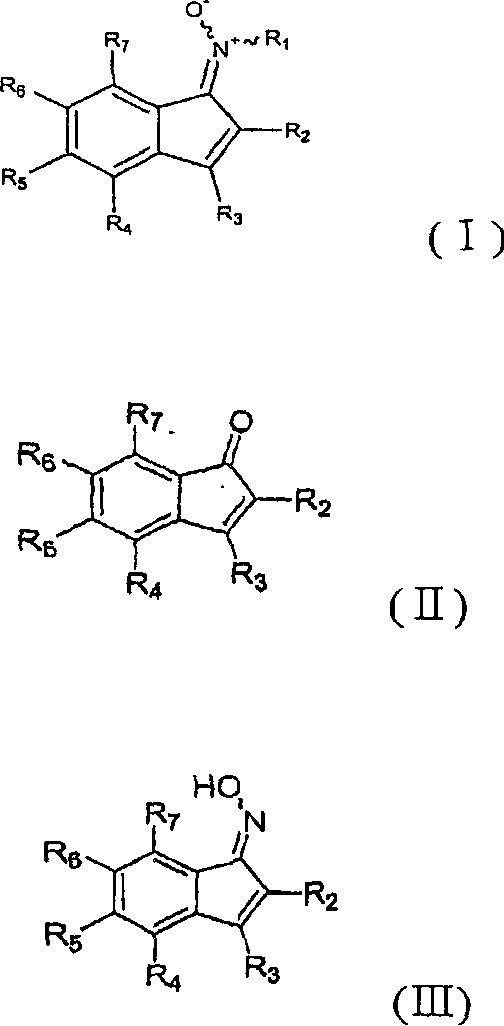
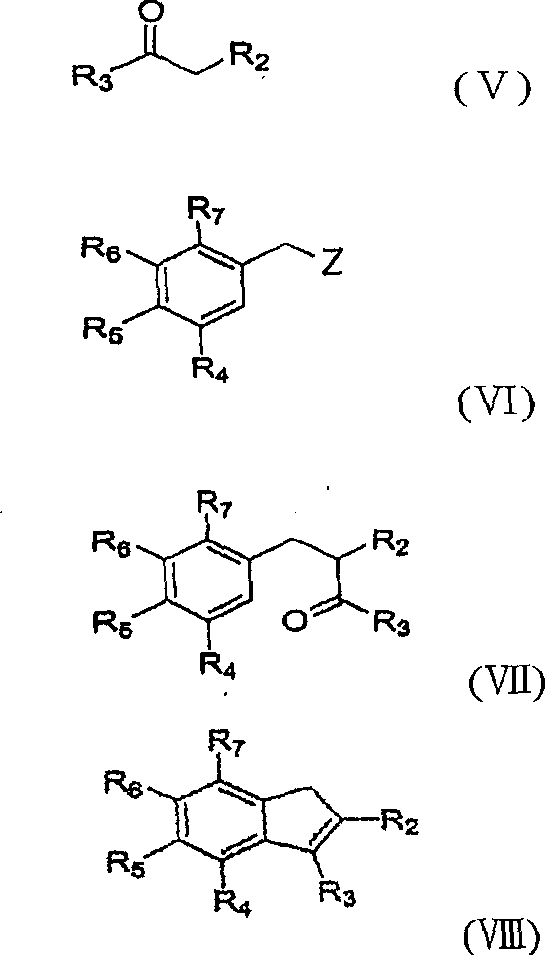
Indene derivatives and process for the preparation thereof
一种茚衍生物、化合物的技术,应用在新型茚衍生物领域,能够解决体重增加等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0214] Embodiment 1: Preparation 1-(trans-methylimino-N-oxygen)-3-phenyl-6-(3-phenylpropoxy)-1H-indene-2-carboxylic acid ethyl ester (table 1 Compound No. 9)
[0215] (step 1) prepare 3-hydroxybenzyl chloride (formula (VI))
[0216] 3-Hydroxybenzyl alcohol (5g, 40mmol) and triethylamine (5.2ml, 60mmol) were dissolved in benzene (250ml), and thionyl chloride (5.2ml) dissolved in benzene (50ml) was added thereto at 0°C . The brownish reaction solution was stirred at room temperature for 6 hours. When the reaction was complete, the solution was washed with brine, and the aqueous layer was extracted with dichloromethane. The organic extract was dried over anhydrous magnesium sulfate and concentrated under reduced pressure to obtain the title compound (5.7 g, 99%).
[0217] 1 H NMR (CDCl 3 , 200MHz) δ7.22(t, J=7.7Hz, 1H), 6.96-6.78(m, 3H), 5.73(s, 1H), 4.52(s, 2H)
[0218] (Step 2) Preparation of ethyl 2-(3-hydroxybenzyl)-3-oxo-3-phenylpropionate (formula VII)
[0219] Ethy...
Embodiment 2
[0240] Example 2: Preparation of 1-(trans-methylimino-N-oxygen)-6-(2-morpholin-4-ylethoxy)-3-phenyl-1H-indene-2-carboxylic acid Ethyl ester (Compound No. 33 of Table 1)
[0241] (Step 1) Preparation of ethyl 3-phenyl-6-(2-morpholin-4-ylethoxy)-1-oxo-1H-indene-2-carboxylate [compound of formula (II)] (reaction Diagram (VII))
[0242] 6-Hydroxy-1-oxo-3-phenyl-1H-indene-2-carboxylic acid ethyl ester [compound of formula (II)] (10.90 g, 26.75 mmol) prepared in step 4 of Example 1 was dissolved in Tetrahydrofuran: benzene (270ml: 90ml). Subsequently, 4-(2-hydroxyethyl)morpholine (5.83 g, 44.45 mmol) and triphenylphosphine (11.66 g, 44.45 mmol) were added thereto. Diisopropyl azodicarboxylate (8.99 g, 44.45 mmol) was added dropwise to the mixture at 0°C, and stirred at room temperature for 2 hours. The reaction mixture was washed with saturated sodium chloride and extracted with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, concentrated, and the r...
Embodiment 3
[0246] Example 3: Preparation of ethyl 1-(trans-methylimino-N-oxygen)-5,6-methylenedioxy-1-oxo-3-phenyl-1H-indene-2-carboxylate (Compound No. 48 in Table 1)
[0247] (Step 1) Preparation of 5-chloromethylbenzo[1,3]dioxole [compound of chemical formula (VI)]
[0248] Piperonyl alcohol (10 g, 65.7 mmol) was dissolved in benzene. Triethylamine (11 ml, 78.8 mmol) and thionyl chloride (11 ml, 131.4 mmol) were added dropwise thereto and stirred at 0° C. for 24 hours. The reaction mixture was extracted with sodium bicarbonate and ethyl acetate, and the organic layer was separated and dried over anhydrous magnesium sulfate to obtain 5-chloromethylbenzo[1,3]dioxole (11.2 g, yield 100 %).
[0249] 1 H NMR (200MHz, CDCl 3 ): δ6.88-6.75(m, 3H), 5.97(s, 2H), 4.53(s, 2H).
[0250] (Step 2) Preparation of ethyl 2-benzo[1,3]dioxol-5-ylmethyl-3-oxo-3-phenylpropanoate [compound of formula (VII)]
[0251] 5-Chloromethylbenzo[1,3]dioxole (11.2 g, 65.7 mmol) was dissolved in dimethylformami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com