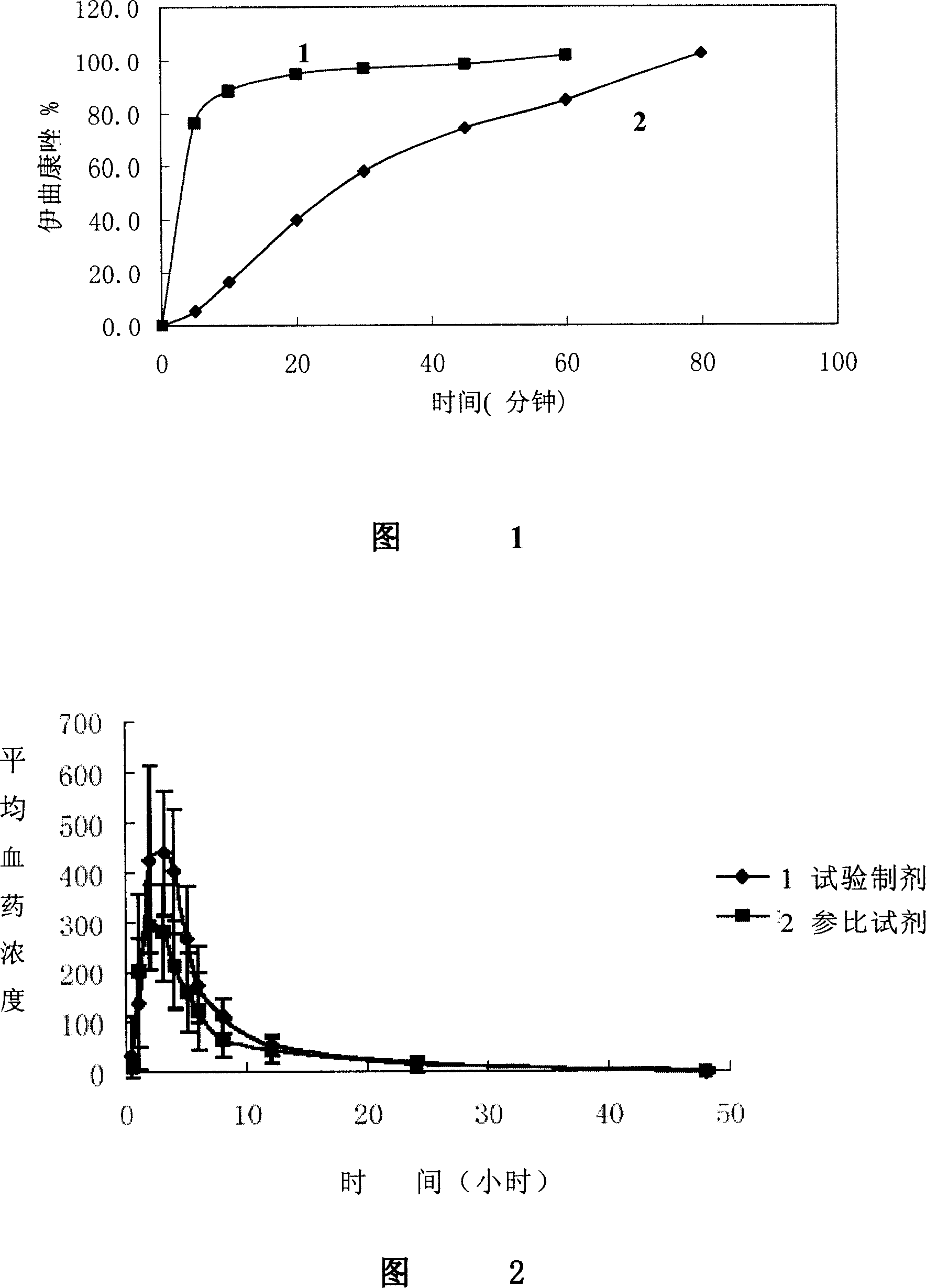
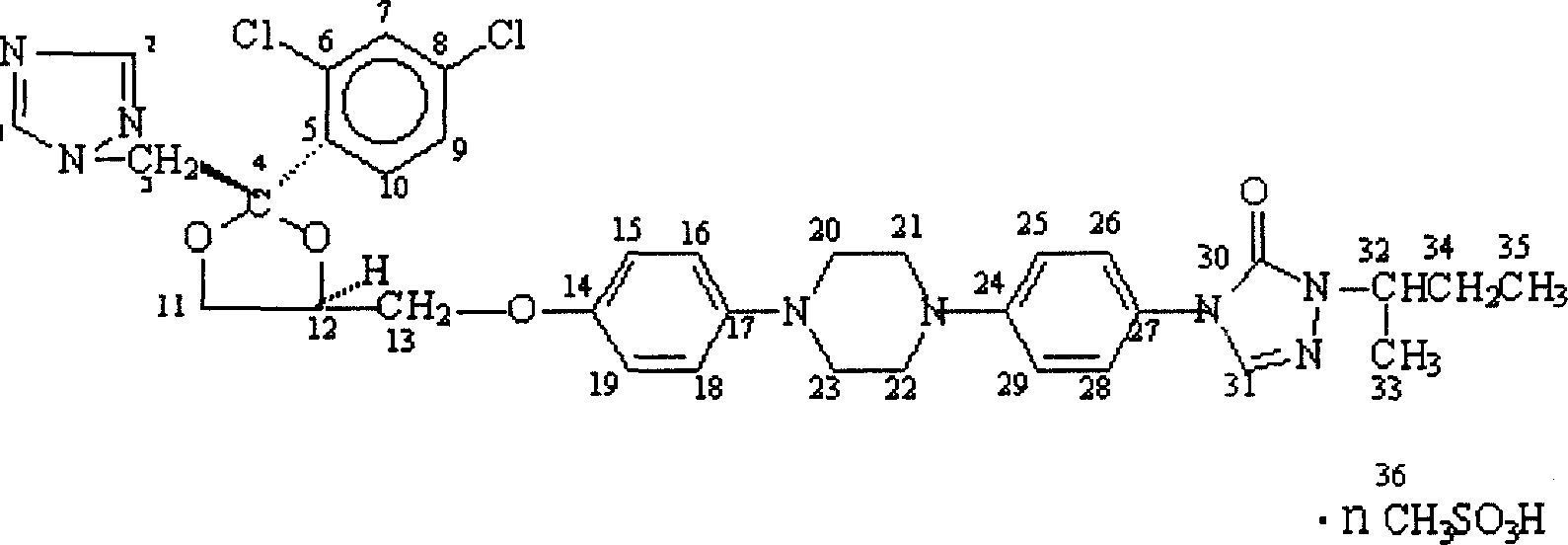
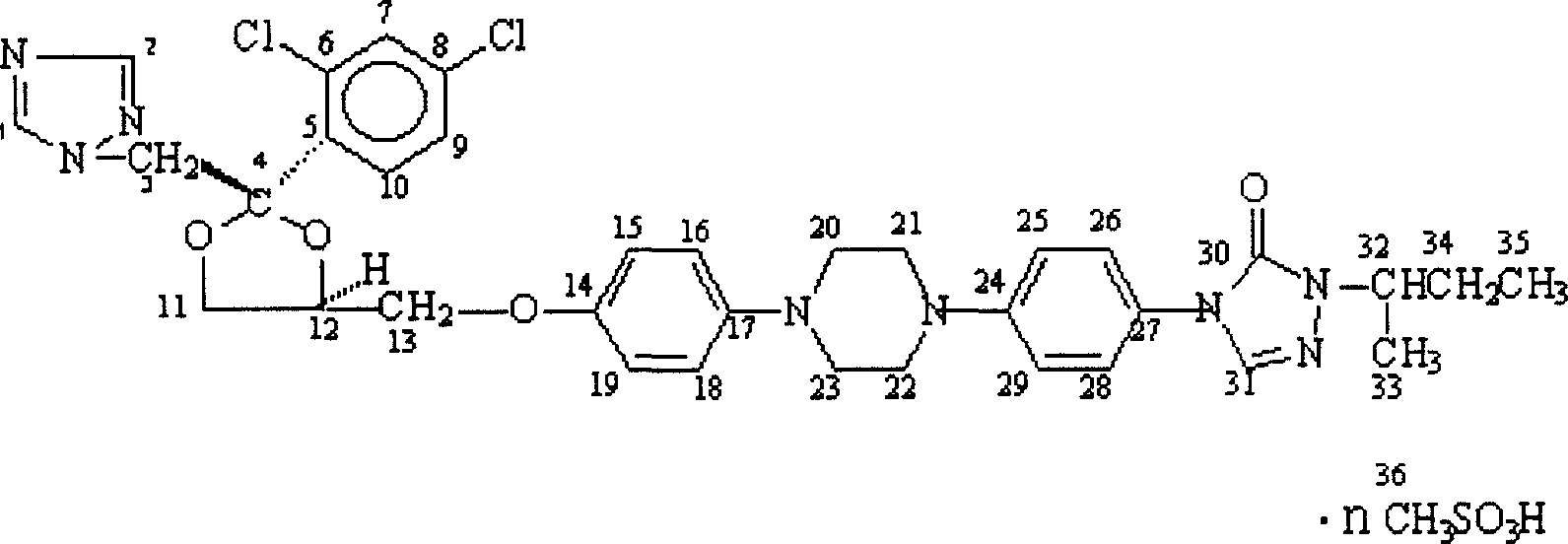
Itraconazole mesylate and its composition and preparation method
A technology of itraconazole mesylate and composition, applied in the field of itraconazole salt and preparation thereof, can solve the problems of unfavorable drying, surface layer becoming pasty, and high equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] These pharmaceutical compositions can be prepared by mixing with suitable pharmaceutical additives such as excipients, disintegrants, binders, lubricants, diluents, buffers or by diluting and dissolving in suitable additives , isotonic agent, preservative, wetting agent, emulsifier, dispersant, stabilizer, solubilizer, etc., and prepare the pharmaceutical composition according to conventional methods.
[0056] When the pharmaceutical composition of the present invention is used for actual treatment, the dose of the compound represented by the above general formula as the active ingredient can be appropriately determined according to the age, sex, body weight, symptoms and degree of treatment of each patient. The dose is approximately 0.1-1,000 mg per adult per day, and the dose is approximately 0.01-500 mg per adult per day for parenteral administration, and the daily dose can be divided into one or several times a day and administered at an appropriate time .
[0057]...
Embodiment 1
[0065] Preparation of itraconazole dimesylate
[0066] Add 50g of itraconazole and 600ml of acetone into a 1L reaction flask, add excess methanesulfonic acid under reflux and stirring, after the reaction is complete, filter with suction, wash with acetone, and dry at 60°C to obtain itraconazole methanesulfonic acid Salt 60.3g, yield 95%.
[0067] Elemental Analysis C 35 h 38 N 8 o 4 .2CH 3 SO 3 H: (exp. / calc.) C 49.33 / 49.49, H 5.18 / 5.16, N 12.56 / 12.48, Cl 7.83 / 7.90, S 7.19 / 7.14.
[0068] Itraconazole mesylate 1 H-NMR data (using deuterated dimethyl sulfoxide as solvent)
[0069]
[0070] 1 Information about H-NMR spectrum
[0071] proton number
Embodiment 2
[0073] Preparation of itraconazole dimesylate
[0074] Add 2g of itraconazole and 20ml of ethanol into a 50ml reaction bottle, heat to reflux, add excess methanesulfonic acid under stirring, after the reaction is complete, cool to room temperature, filter, wash with ethanol, and dry to obtain itraconazole Azole mesylate 2.36g. Yield 93%.
[0075] elemental analysis and 1 The HNMR data were the same as shown in Example 1, indicating the formation of itraconazole dimesylate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com