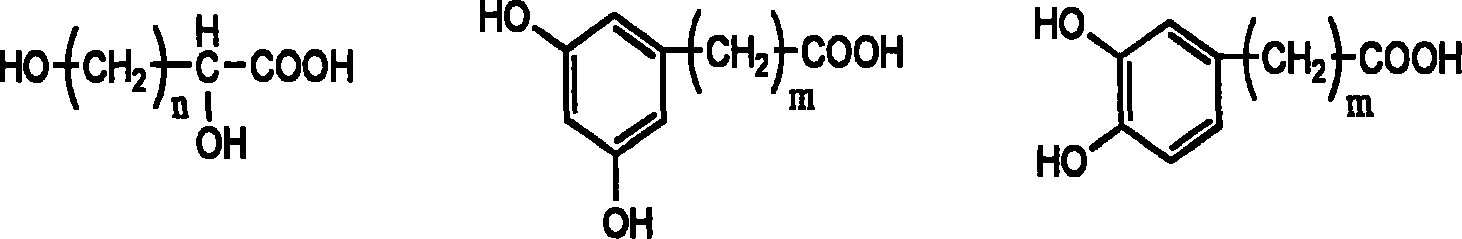
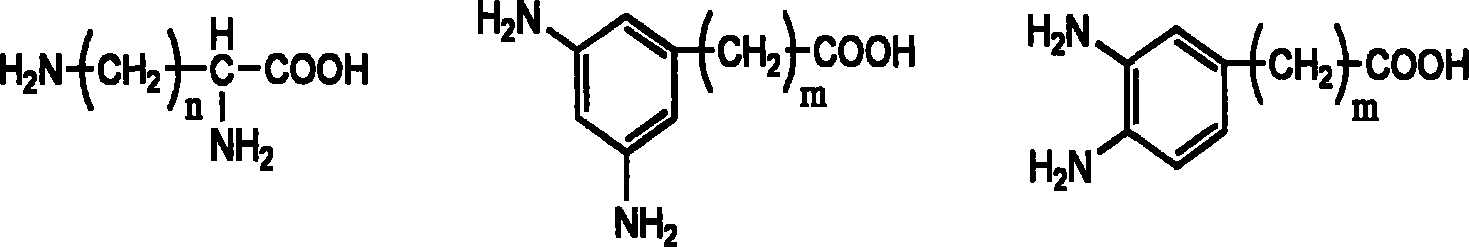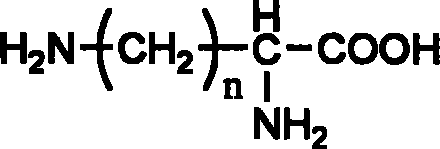Multiple-arm polyethylene glycol with functional group at long chain end, its preparation method and uses
A technology of multi-arm polyethylene glycol and polyethylene glycol, which is applied in the field of multi-arm polyethylene glycol and its preparation, can solve problems such as difficulty in controlling the quality of conjugates, achieve high biological activity and reduce immune response Effects that occur and increase the chance of collision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] mPEG with different arm lengths 3 -Lys-PEG-NH 2 Preparation (p-nitrophenol ester activation method)
[0034] Dissolve lysine (439 mg, 3 mmol) in 20 ml of water with a pH value of 8.0 to 8.3, and then add mPEG to it in batches within 3 hours 2 -Lys-CO 2 PhNO 2 (two-arm mPEG p-nitrophenol ester, molecular weight 10000, wherein one arm is 7000, the other arm is 3000, 10g, 1mmol), while maintaining the pH value of the system at 8.3 with 0.2mol / L NaOH. After stirring overnight at room temperature, the reactant was cooled to 0° C., and the pH value of the system was adjusted to 3 with 2 mol / L hydrochloric acid. Now use diethyl ether to extract impurities from water, and then use chloroform to continuously extract three times. After the extract is concentrated, it is added dropwise into anhydrous diethyl ether to obtain a white precipitate. 2 Mono-substituted lysine (mPEG 2 -mono-Lysine). The product was characterized by amino titration, GPC and MALDI, and its purity re...
Embodiment 2
[0038] mPEG with different arm lengths 3 -Lys-PEG-NH 2 Preparation (N-hydroxysuccinimide ester activation method)
[0039] It is enough to change the p-nitrophenol in the above-mentioned embodiment 1 into an equivalent amount of N-hydroxysuccinimide.
Embodiment 3
[0041] mPEG with different arm lengths 4 -Lys-PEG-NH 2 Preparation (p-nitrophenol ester activation method)
[0042] To be dissolved with the product mPEG described in embodiment 1 2 - Mono-Lysine (molecular weight 10000, 9g, 0.9mmol) in anhydrous dichloromethane (20mL), add triethylamine (TEA) until the pH value reaches 8. mPEG 2 CO 2 PhNO 2 (Molecular weight 10000, one arm is 6000, the other arm is 4000, 10.5g, 1.05mmol) was added to the reaction solution in batches within three hours, while maintaining the pH value of the system at about 8 with TEA. After reflux for 72 hours, the reactant was cooled to room temperature, concentrated, filtered, precipitated with ether, and then recrystallized with a small amount of ethanol. excess mPEG 2 -CO 2 PhNO 2 After being stirred overnight in a buffer solution with a pH value of 9-10, it is hydrolyzed, the solution is cooled to 0°C, and the pH value is adjusted to 3 with 2mol / L hydrochloric acid system. The p-nitrophenol in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com