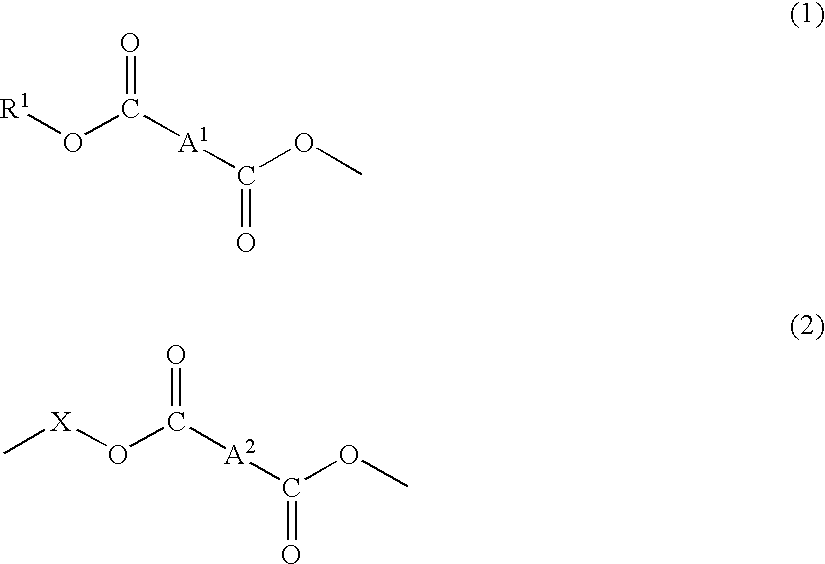
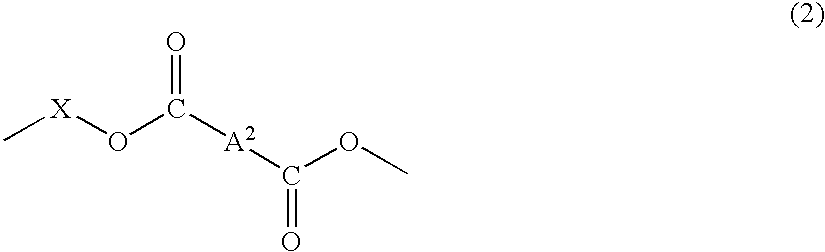
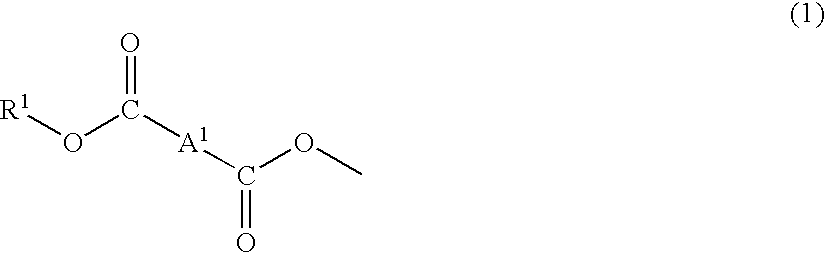Material for plastic lens, production process of the material, composition for plastic lens, plastic lens obtained by curing the composition, and production process of the plastic lens
a technology of plastic lens and composition, which is applied in the direction of optics, instruments, optical elements, etc., can solve the problems of low resistance to solvent or scratching, insufficiently high value of plastic lens with respect to birefringence and light scattering, and high refractive index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
[0118] Into a 500 ml three-neck flask with a distillation unit, 160.2 g of dimethyl glutarate, 232.3 g of allyl alcohol, 0.27 g of potassium acetate and 1.33 g (1% by mass based on dimethyl glutarate) of calcium hydroxide were charged. The system was heated at a bath temperature of 110 to 120.degree. C. in a nitrogen stream, and methanol generated was distilled off. The reaction was continued until a theoretical amount of methanol was distilled off, and when 64 g (100% based on the theoretical distillation amount of methanol) was distilled off, the reactor was cooled. The resulting reaction solution was purified by distillation under reduced pressure, as a result, 200 g of diallyl glutarate was obtained as a colorless transparent liquid (isolation yield: 95%, raw material: 1 mol).
[0119] Into a 300 ml three-neck flask with a distillation unit, 101 g (0.48 mol) of diallyl glutarate, 53.8 g (0.373 mol) of 1,4-cyclohexane dimethanol and 0.1 g (1% by mass based on diallyl glutarate) of d...
production example 2
[0123] Into a 3 l three-neck flask with a distillation unit, 660.6 g of succinic anhydride, 1,056 g of allyl alcohol, 1,000 ml of benzene and 6.61 g (1% by mass based on succinic anhydride) of concentrated sulfuric acid were charged. The system was heated at 100.degree. C. in a nitrogen stream, and H.sub.2O generated was distilled off. The reaction was continued until a theoretical amount of H.sub.2O was distilled off and when 109 g (100% based on the theoretical distillation amount of H.sub.2O) of H.sub.2O was distilled off, the reactor was cooled. The resulting reaction solution was neutralized with an aqueous NaOH solution and washed with water. After benzene and excess allyl alcohol were distilled off, the reaction solution was purified by distillation under reduced pressure, as a result, 1,130 g of diallyl succinate was obtained as a colorless transparent liquid.
[0124] Into a 3 l three-neck flask with a distillation unit, 1,784 g of diallyl succinate, 829 g of 1,4-cyclohexanedi...
production example 3
[0128] Into a 1 l three-neck flask with a distillation unit, 1,000 g of dimethyl 2-methylsuccinate, 1,441 g of allyl alcohol, 25 g of potassium acetate and 5 g of calcium hydroxide were charged. The system was heated at 110.degree. C. in a nitrogen stream, and methanol generated was distilled off. The reaction was continued until a theoretical amount of methanol was distilled off and when 385.7 g (97%) was distilled off, the reactor was cooled. The resulting reaction solution was filtered by suction using a No. 5C Kiriyama funnel to separate the solid matters in the reaction solution. Thereafter, the solution was purified by distillation under reduced pressure, as a result, 1,200 g of diallyl 2-methylsuccinate was obtained as a colorless transparent liquid.
[0129] Into a 1 l three-neck flask with a distillation unit, 640 g of diallyl 2-methylsuccinate, 336 g of 1,4-cyclohexanedimethanol and 0.639 g of dibutyltin oxide were charged. The system was heated at 180.degree. C. in a nitroge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| curing shrinkage | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com