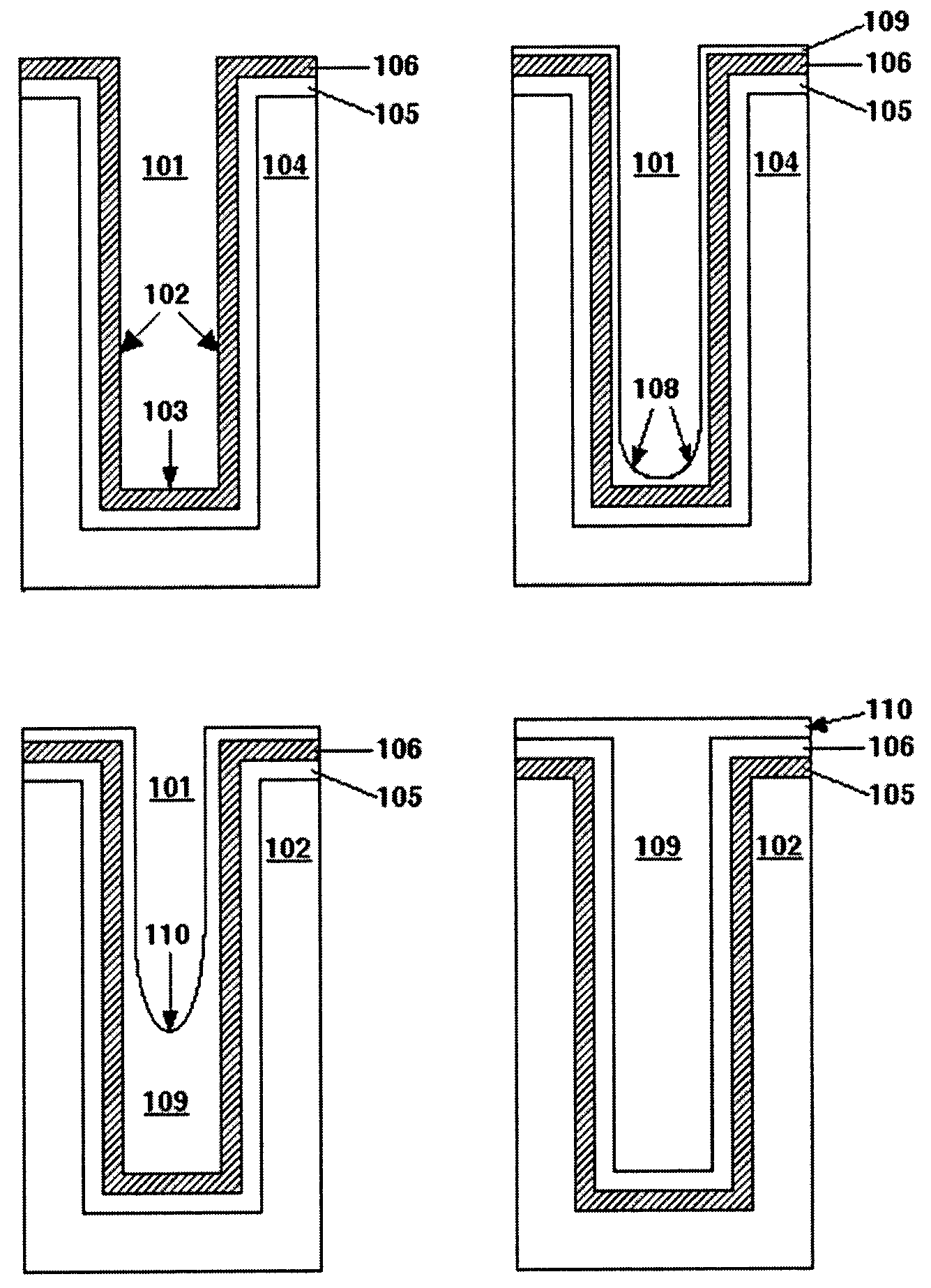
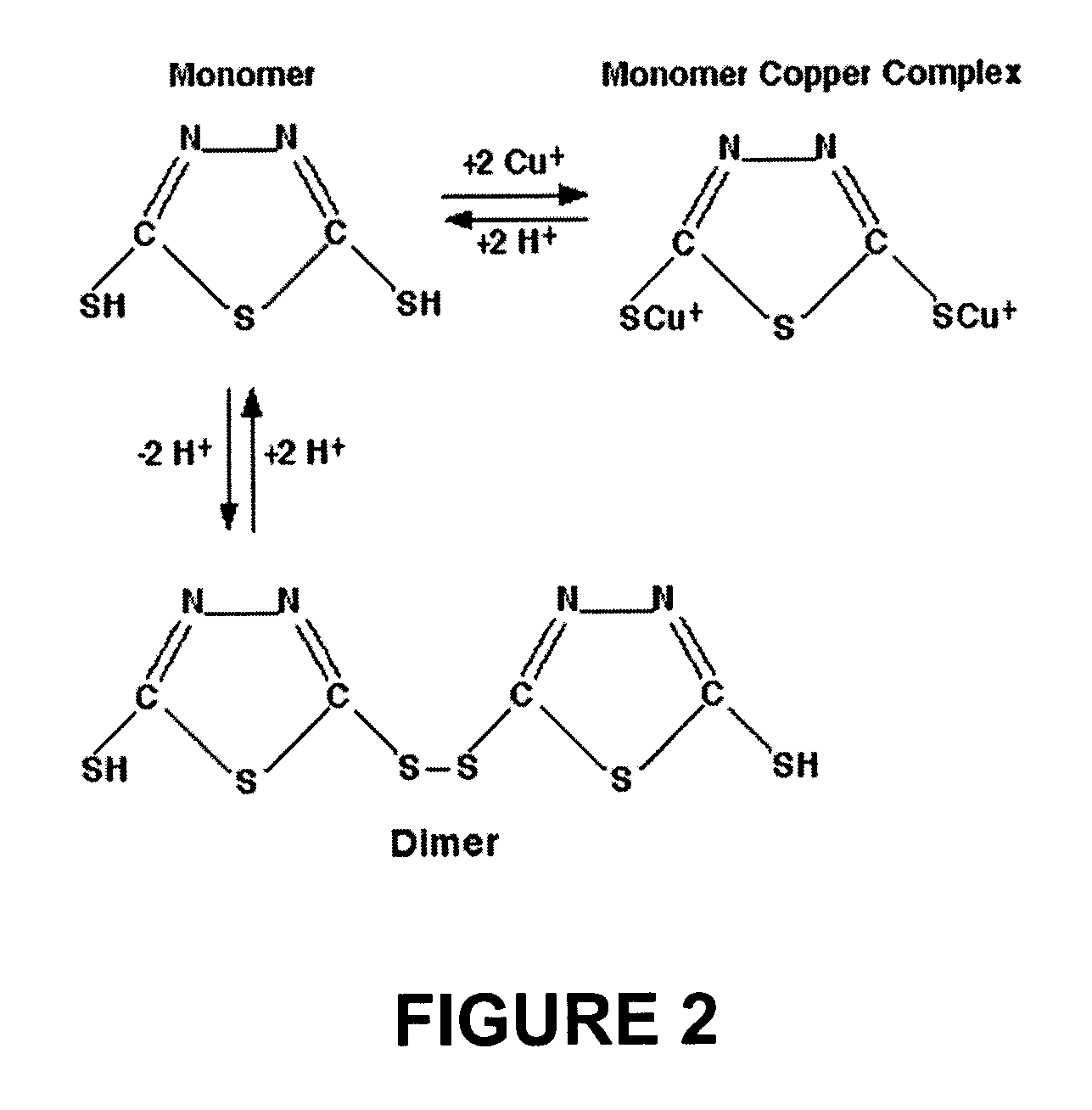
Copper bath for electroplating fine circuitry on semiconductor chips
a technology of fine circuitry and copper bath, which is applied in the manufacture of printed circuits, basic electric elements, electric apparatus, etc., can solve the problems of difficult to achieve close control, complex additive system, and inability to accept damascene copper deposits, so as to prevent runaway copper electrodeposition, improve the deposit properties, and avoid interference from breakdown products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
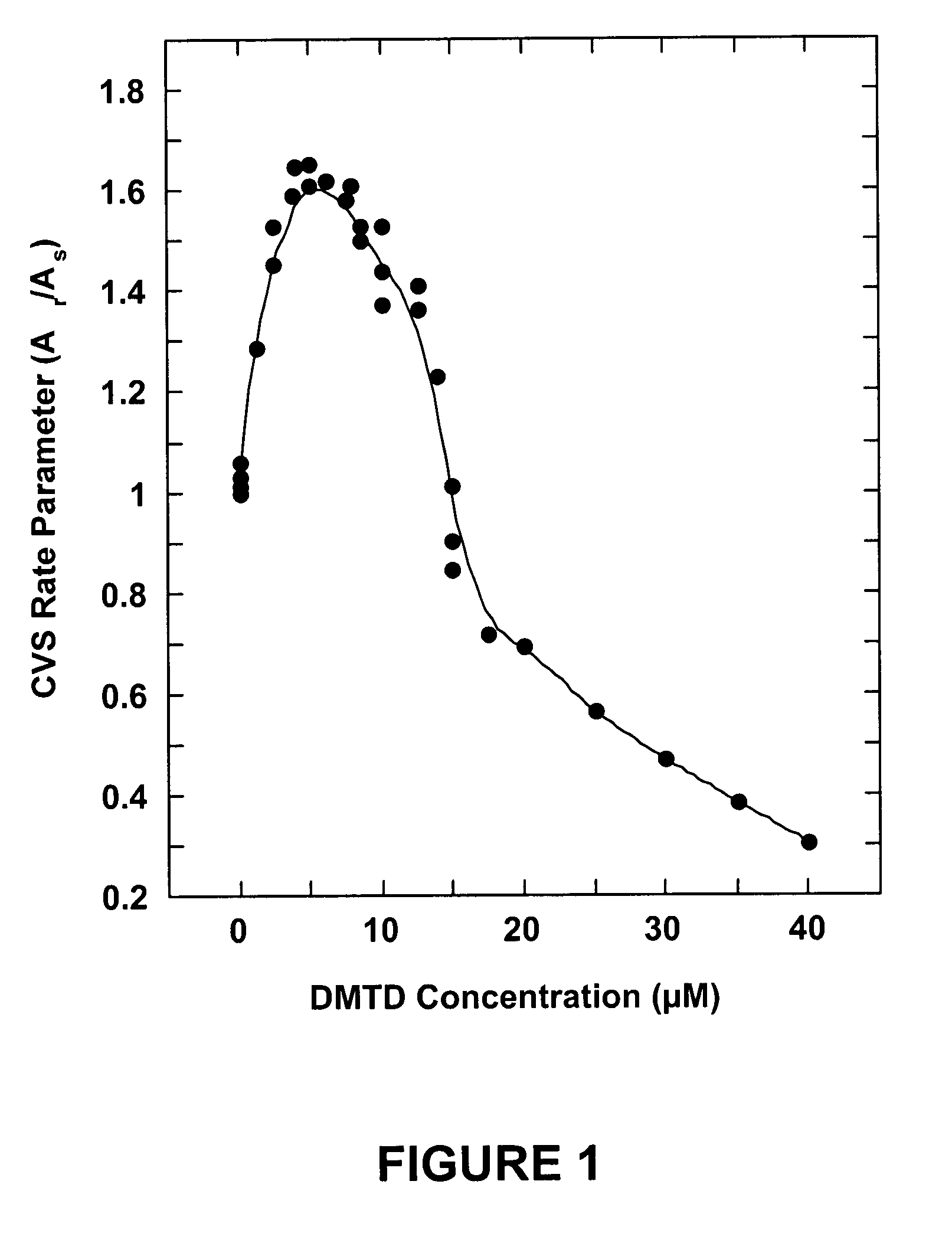
[0058] Coupons were plated from copper pyrophosphate solutions (55° C.) containing 0.0, 0.33, 0.67, 1.0, 1.3, 1.7, 2.0, 2.3, 3.3, 5.0 or 8.3 μM of the DMTD additive (without surfactant added). Good bottom-up filling of the smallest features (0.13 μm vias) was attained with the 2.0 μM solution, as indicated by the micrograph in FIG. 6. Good bottom-up filling of all of the larger features (0.20, 0.33 and 0.38 μm wide) was attained with the 1.3, 1.7 and 2.0 μM solutions. Incomplete filling was observed for all features with all of the other solutions, except that good bottom-up filling was observed for the two larger features (0.33 and 0.37 μm wide) with the 3.3 μM solution. These data show that the DMTD monomer additive of the present invention provides good bottom-up filling of Damascene features as fine as 0.13 μm wide (5.4:1 aspect ratio). The optimum DMTD concentration was about 2 μM for the conditions used but would be expected to depend on the plating parameters, such as current...
example 2
[0059] Coupons were plated from copper pyrophosphate solutions (55° C.) containing 0.50 g / L Triton®-X surfactant and 0.0, 0.33, 0.67, 1.0, 1.3, 1.7, 2.0, or 5.7 μM of the DMTD additive. As was the case without surfactant added, a DMTD concentration of about 2 μM provided good bottom up filling of even the smallest Damascene features (0.13 μm vias) in the presence of the Triton®-X surfactant.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com