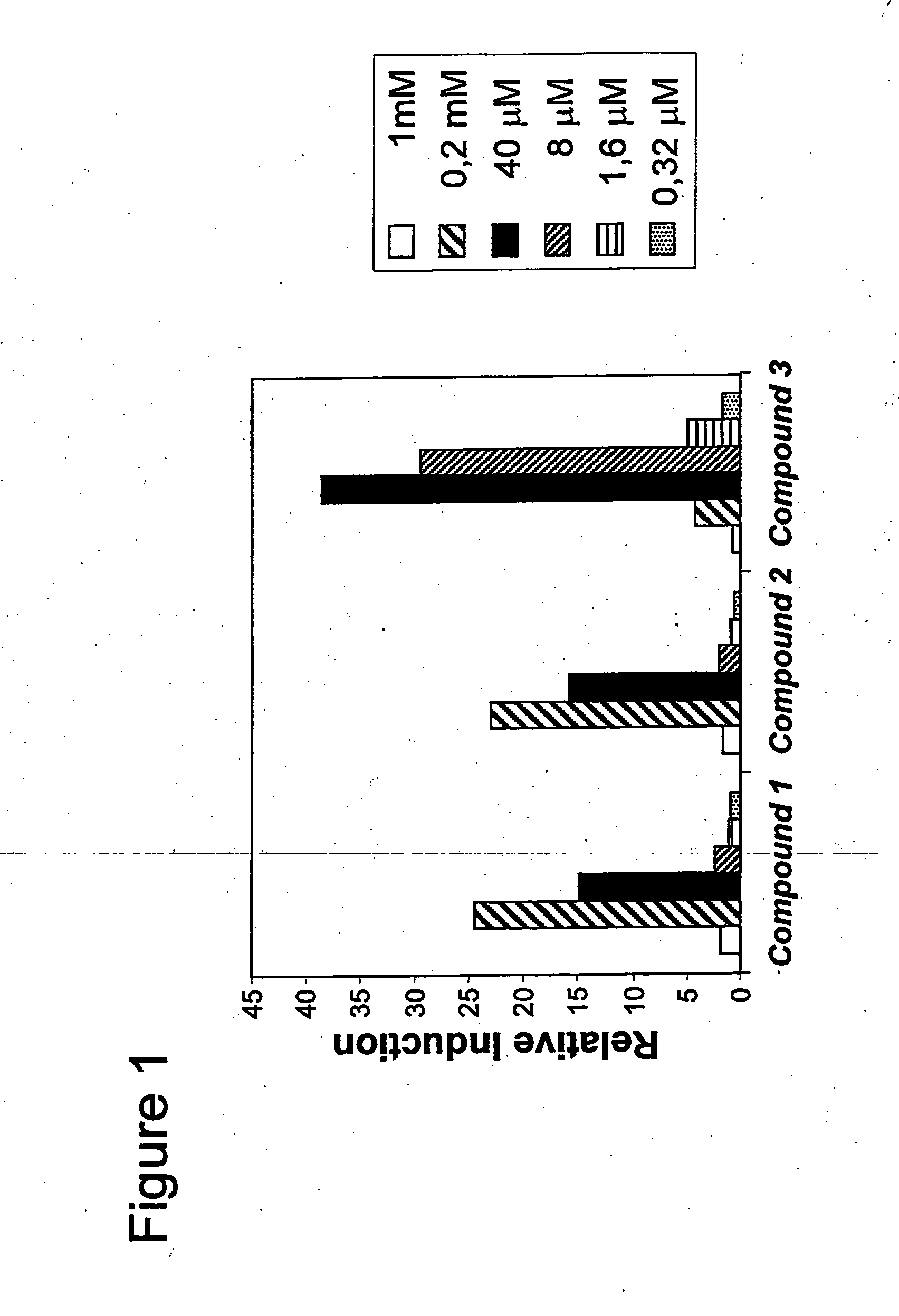
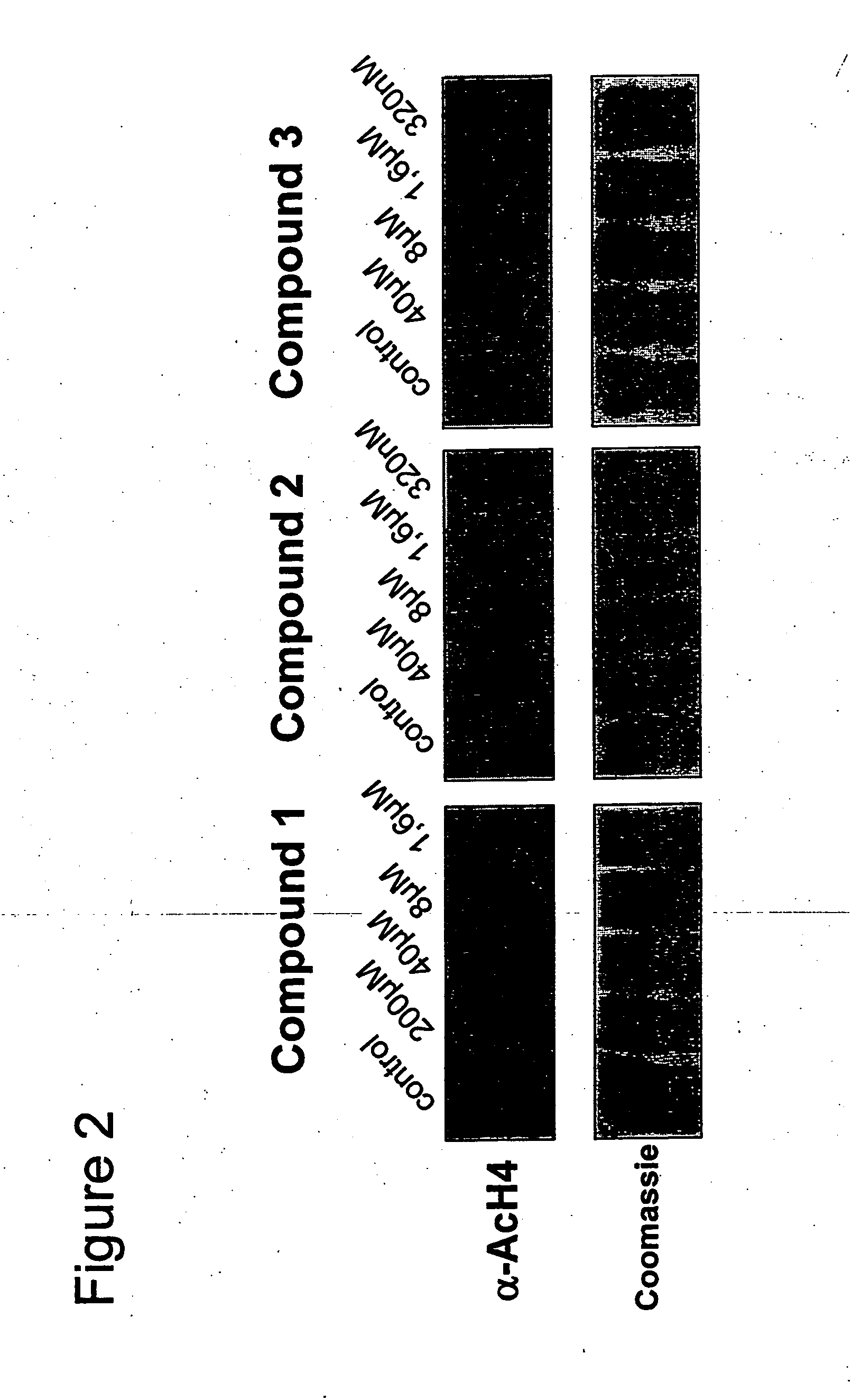
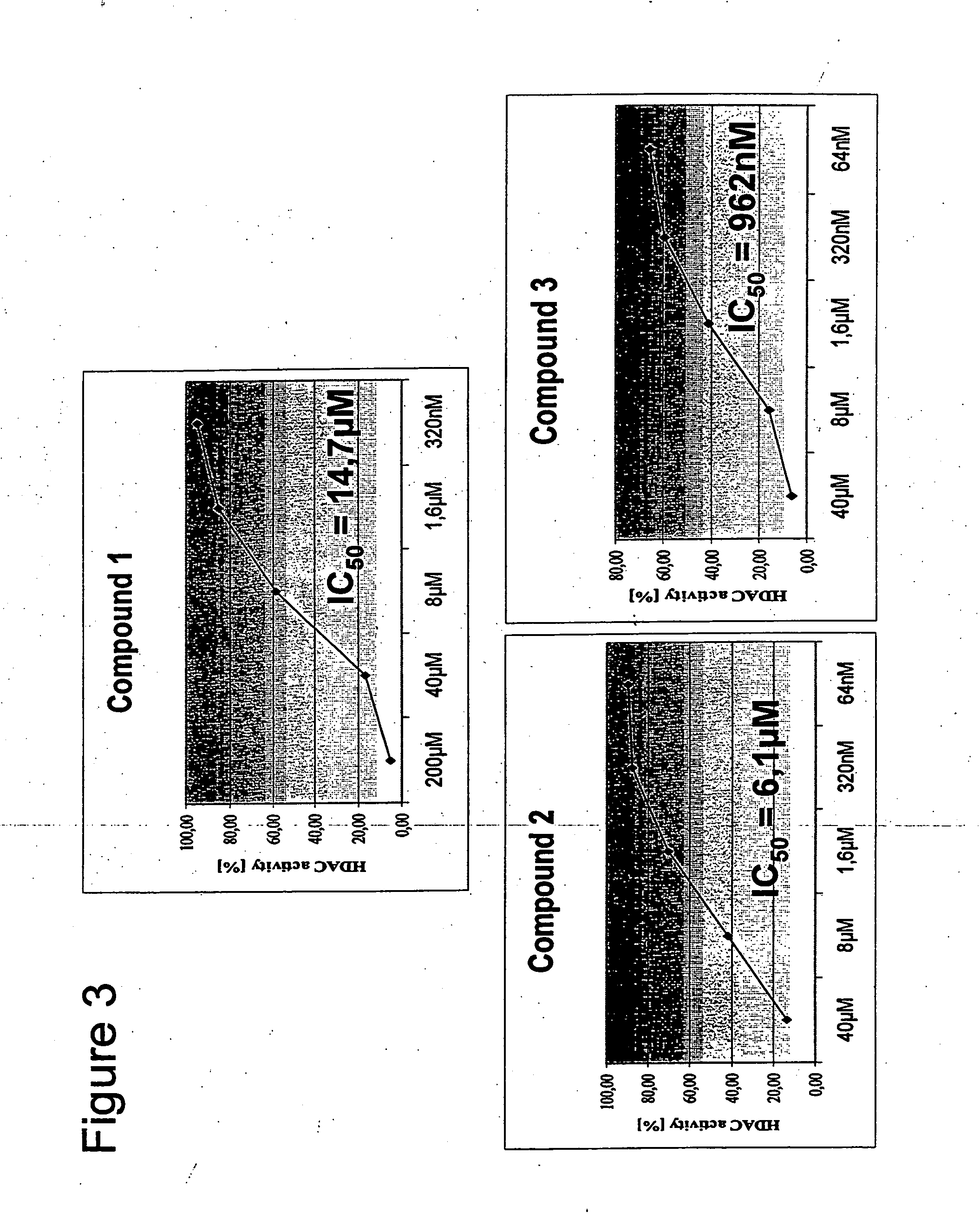
Novel compounds as histone deacetylase inhibitors
a technology of histone deacetylase and compounds, which is applied in the field of new compounds as histone deacetylase inhibitors, can solve the problems of tumorigenic transformation, decrease of therapeutic doses, and destabilisation of the interaction of histones with dna, and achieve the effect of improving the condition of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Compound 1 (3-Cyclopentyl-N-hydroxy-propionamide)
[0085] To a solution of 3-Cyclopentyl-propionyl chloride (11.0 mL, 11.6 g, 72.0 mmol) in dichloromethane (50 mL) hydroxylamine hydrochloride (5.00 g, 72.0 mmol) and sodium bicarbonate (12.0 g, 144 mmol) were added. After stirring for 24 h at room temp. the reaction was quenched by addition of saturated ammonium chloride solution (50 mL). The layers were separated. The water layer was extracted with ethyl acetate (4×50 mL). The solvent of the combined organic layers was removed in vacuo. Precipitation of the crude product out of ethyl acetate by adding petroleum ether yielded the hydroxamate (5.25 g, 33.4 mmol, 46%) as a white solid. 1H NMR (300 MHz, [D6-DMSO]: δ=0.74-0.91 (m, 2H), 1.05-1.25 (m, 4H), 1.36 (q, J=7.4 Hz, 4H), 1.54-1.71 (m, 5H), 1.94 (t, J=7.6 Hz, 2H), 8.59 (s,1H) and 10.28 (s, 1H) (NH and OH). 13C NMR (75 MHz, [D6-DMSO]: δ=25.6, 26.0, 29.7, 32.4, 36.5, 169.2 (C(═O)NHOH). MS: m / z calcd for (C8H14NO2) [M+H]+...
example 2
Synthesis of Compound 2 (3-Cyclohexyl-N-hydroxy-propionamide):
[0086] To a solution of 3-Cyclohexyl-propionyl chloride (12.1 mL, 12.6 g, 72.0 mmol) in dichloromethane (50 mL) hydroxylamine hydrochloride (5.00 g, 72.0 mmol) and sodium bicarbonate (12.0 g, 144 mmol) were added. After stirring for 24 h at room temperature the reaction was quenched by addition of saturated ammonium chloride solution (50 mL). The layers were separated. The water layer was extracted with ethyl acetate (4×50 mL). The solvent of the combined organic layers was removed in vacuo. Precipitation of the crude product out of ethyl acetate by adding petroleum ether yielded the hydroxamate (7.21 g, 42 mmol, 58%) as a white solid. 1H-NMR (300 MHz, [D6-DMSO]: δ=0.95-1.12 (m, 2H), 1.38-1.69 (m, 6H), 1.62-1.77 (m, 3H), 1.94 (t, J=7.6 Hz, 2H), 8.58 (s, 1H) and 10.28 (s,1H) (NH and OH); 13C-NMR (75 MHz, [D6-DMSO]: δ=25.0, 31.8, 32.0, 32.3, 39.5, 169.5 (C(═)NHOH); MS: m / z calc. for (C9H17NO2) [M+H]+172; found 172.
example 3
Synthesis of Compound 3 (3-Cyclohexyl-N-hydroxy-acrylamide)
[0087] 3-Cyclohexyl-acrylic acid ethyl ester: to a cooled solution of oxalyl chloride (11.1 mL, 105 mmol) in dichloromethane (250 mL) was added at −78° C. a solution of dimethylsulfoxide (14.6 mL, 206 mmol) in dichloromethane (250 mL). After 5 min at the same temperature cyclohexylmethanol (10.8 mL, 87.5 mmol) and after additional 5 min triethylamine (60.7 mL, 438 mmol) were added. The reaction remained at −78° C. for two hours and then let warm to room temperature. The solvent was removed in vacuo. The resulting aldehyde was used for the next step without further purification. The crude cyclohexanecarbaldehyde was dissolved in toluene (200 mL) and ethanol (150 mL). After 15 min of stirring at 70° C. carbethoxy-methylene triphenylphosphorane (33.6 g, 96.5 mmol) was added in one portion. Stirring was continued for additional 24 hours. The solvent was removed in vacuo. The product was obtained by flash chromatography in 78% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical resistance | aaaaa | aaaaa |
| Gene expression profile | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com