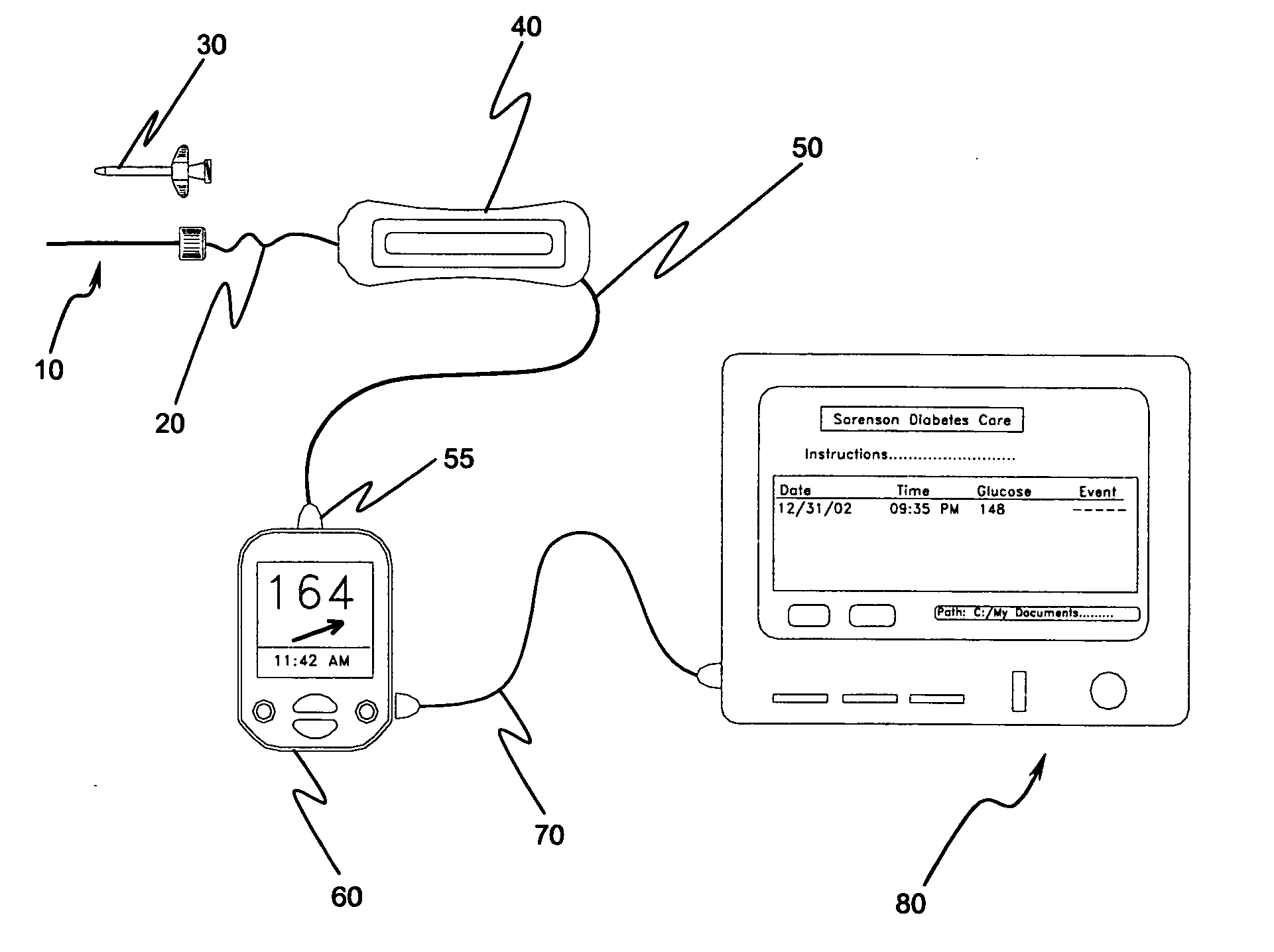
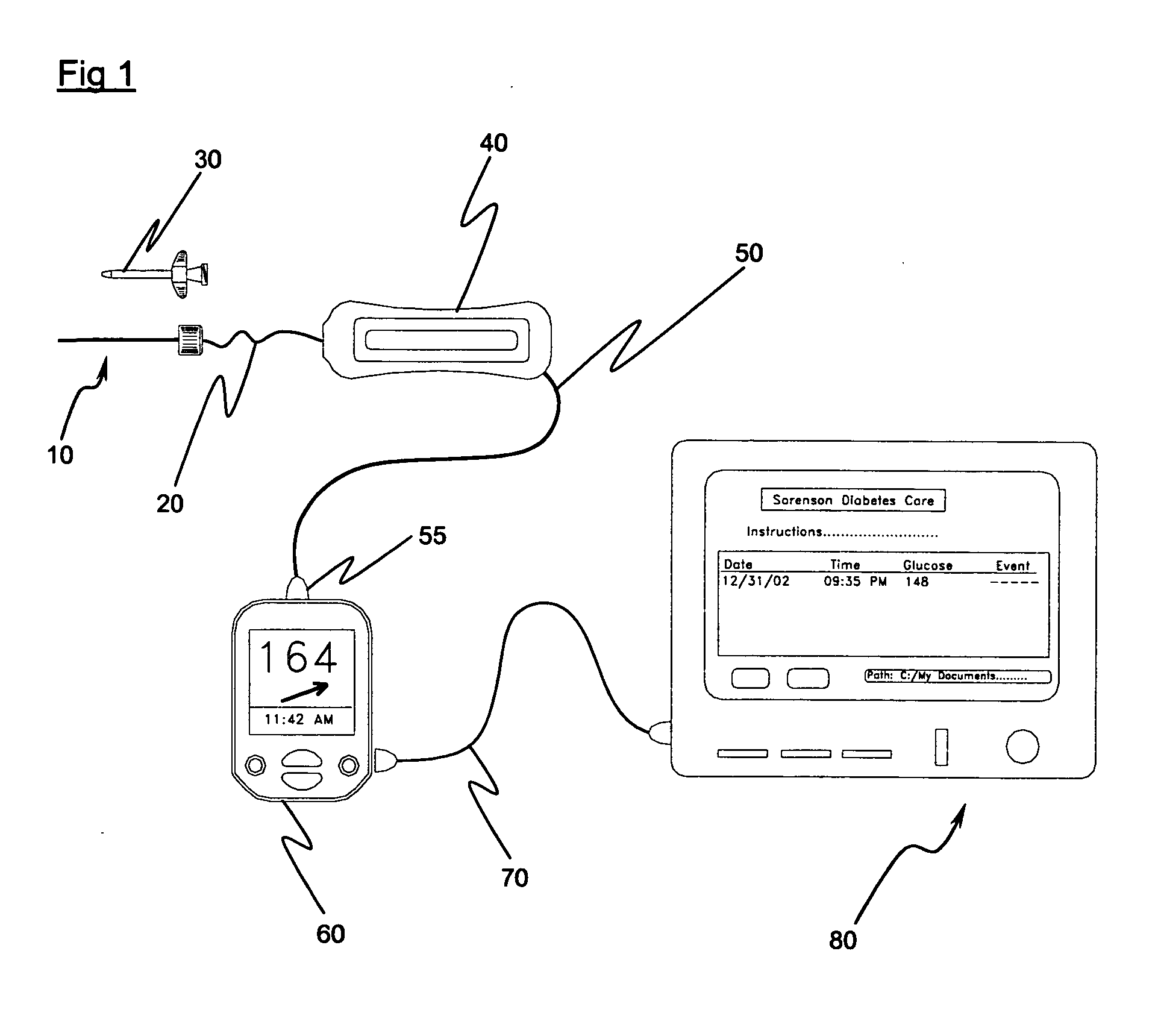
Implantable biosensor system, apparatus and method
a biosensor and implantable technology, applied in the field of medical devices, can solve the problems of inadequate periodic information, less than optimal, and insufficient glucose in urine measurement, and achieve the effect of enhancing the biocompatibility of the biosensor and enhancing the volume of glucose oxidas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
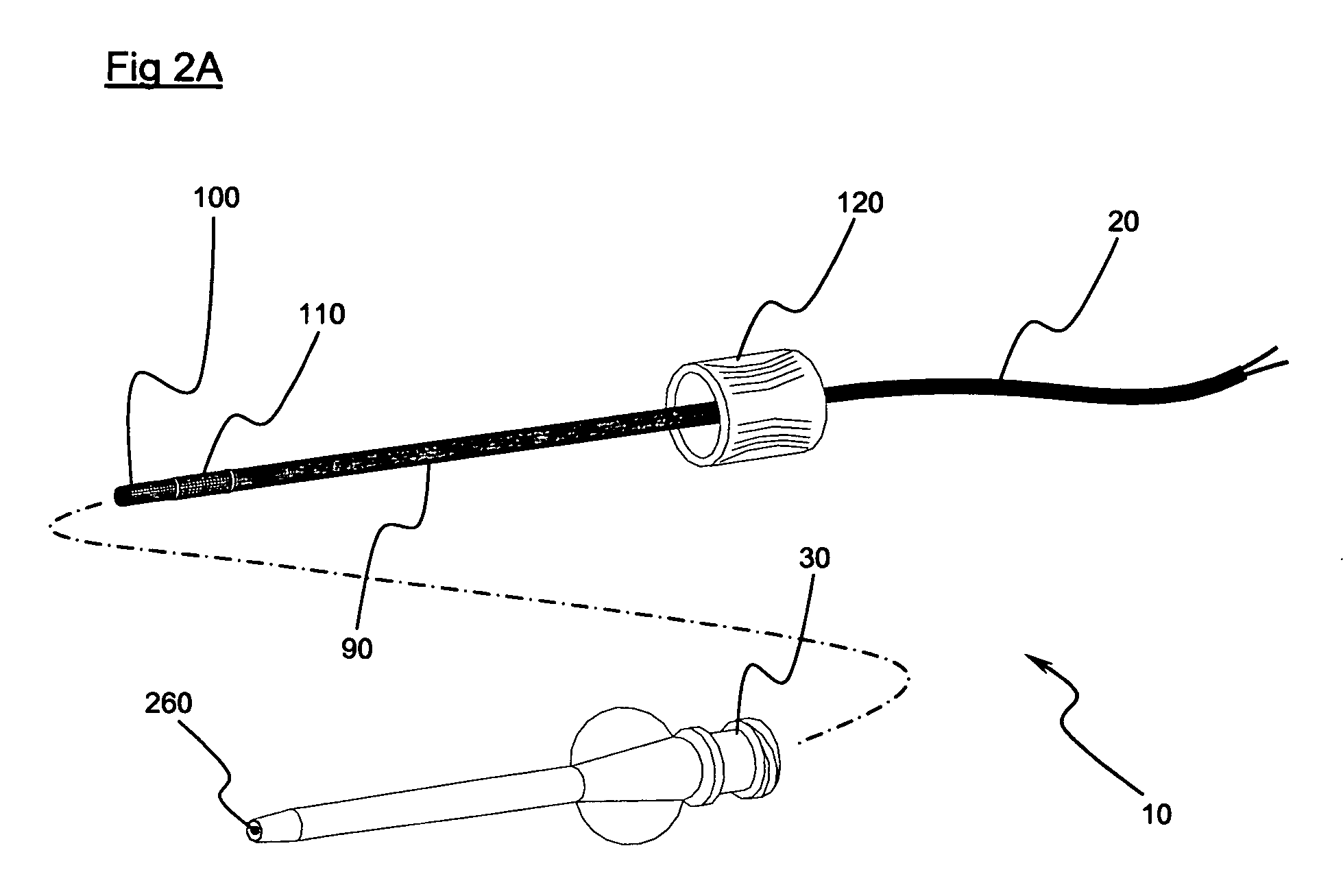
embodiment 10
[0060] The preferred embodiment 10 illustrates the working electrode 100 as being structured in the form of coils. However, it is only necessary that the working electrode 100 be in length substantially not less than the leading portion 280 when the leading portion 280 is laterally deflected to a maximum extent. Such a limitation is operable to resist separation of an electrically conductive path from the electrode due to bending of the biosensor. Correspondingly, whereas in a preferred embodiment the reference electrode 110 is illustrated as being in the form of coils, in essence a working electrode 110 may be in length substantially not less than the trailing portion 290 when the trailing portion 290 is laterally deflected to a maximum extent.
[0061]FIGS. 6A-6D illustrate an alternative preferred embodiment of a biosensor, generally indicated at 330, including an introducer catheter, generally indicated at 340. The biosensor 330 includes a working electrode, generally 350, typicall...
embodiment 330
[0062] The introducer catheter 340, like the introducer catheter 30, includes a lumen that may be thought of as an interior cannula lumen 450. Furthermore, the illustrated introducer catheter 340 presents an advanced end 460 designed for subcutaneous or other intra-tissue placement, an opposite end, generally 470, and a cannula wall 480 defining the interior cannula lumen 450 and comprising an exterior surface 490. The exterior surface 490 and the interior cannula lumen 450 extend between the advanced end 460 and the opposite end 470. In the biosensor embodiment 330, a reference electrode 500 is associated with the exterior surface 490 of catheter 340 in the vicinity of the advanced end 460. Electrode 500 may take other forms, such as a film, band, etching, printed or imprinted layer, or a shell or coating. The interior cannula lumen 450 is of sufficient cross-sectional diameter to pass the biosensor 330. The advanced end 460 and exterior surface 490 of the introducer catheter 340 a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com