Embryonic stem cells and neural progenitor cells derived therefrom
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Derivation of Cell Lines HES-1 and HES-2
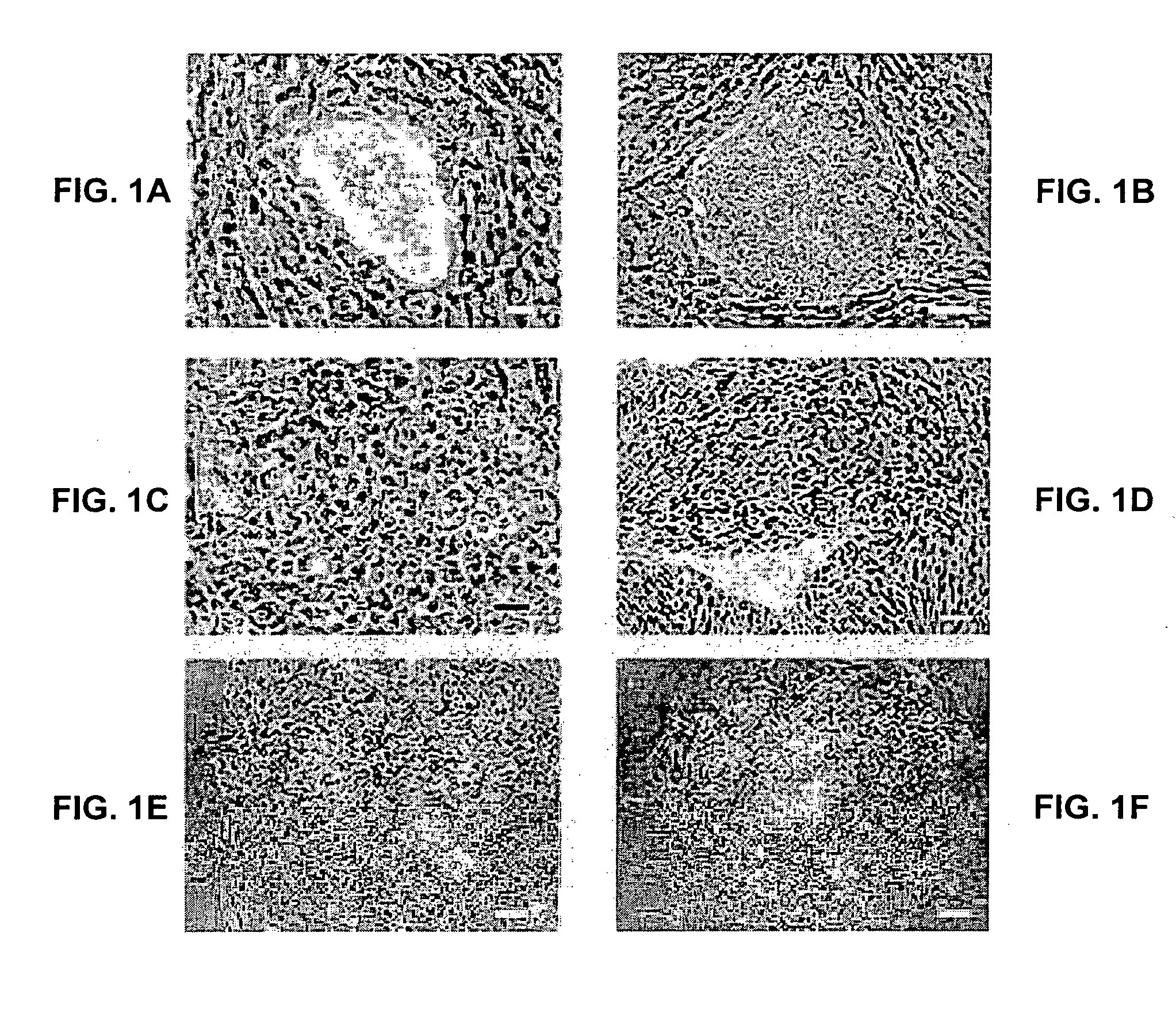
[0400] The outer trophectoderm layer was removed from four blastocysts by immunosurgery to isolate inner cell masses (ICM), which were then plated onto a feeder layer of mouse embryo fibroblasts (FIG. 1A). Within several days, groups of small, tightly packed cells had begun to proliferate from two of the four ICM. The small cells were mechanically dissociated from outgrowths of differentiated cells, and following replating they gave rise to flat colonies of cells with the morphological appearance of human EC or primate ES cells (FIG. 1B, C stem cell colonies). These colonies were further propagated by mechanically disaggregation to clumps which were replated onto fresh feeder cell layers. Growth from small clumps of cells (<10 cells) was not possible under the conditions of these cultures. Spontaneous differentiation, often yielding cells with the morphological appearance of early endoderm, was frequently observed during routine passage of th...
example 2
Marker Expression and Karyotype of the Human ES Cells
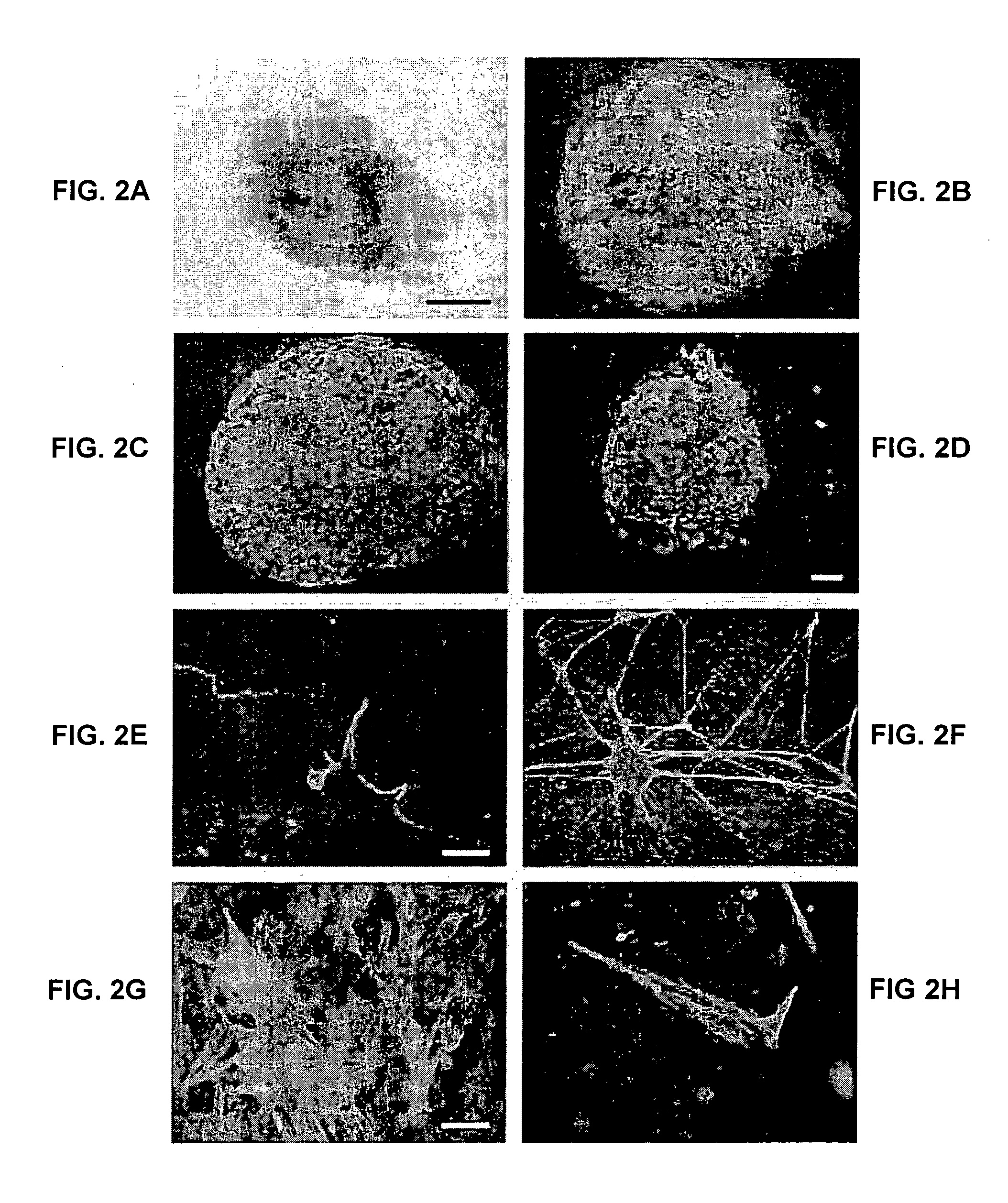
[0401] Marker and karyotype analysis were performed on HES-1 at passage levels 5-7, 14-18, 24-26 and 44-46, and on HES-2 at passage levels 6-8. ES cells contained alkaline phosphatase activity (FIG. 2A). Imiunophenotyping of the ES cells was carried out using a series of antibodies which detect cell surface carbohydrates and associated proteins found on human EC cells. The ES cells reacted positively in indirect immunofluorescence assays with antibodies against the SSEA-4 and TRA 1-60 carbohydrate epitopes, and the staining patterns were similar to those observed in human EC cells (FIG. 2B, 2C). ES cells also reacted with monoclonal antibody GCTM-2, which detects an epitope on the protein core of a keratan sulphate / chondroitin sulphate pericellular matrix proteoglycan found in human EC cells (FIG. 2D). Like human EC cells, human ES cells did not express SSEA-1, a marker for mouse ES cells. Both cell lines were karyotypically norm...
example 3
Differentiation of Human ES Cells in vitro
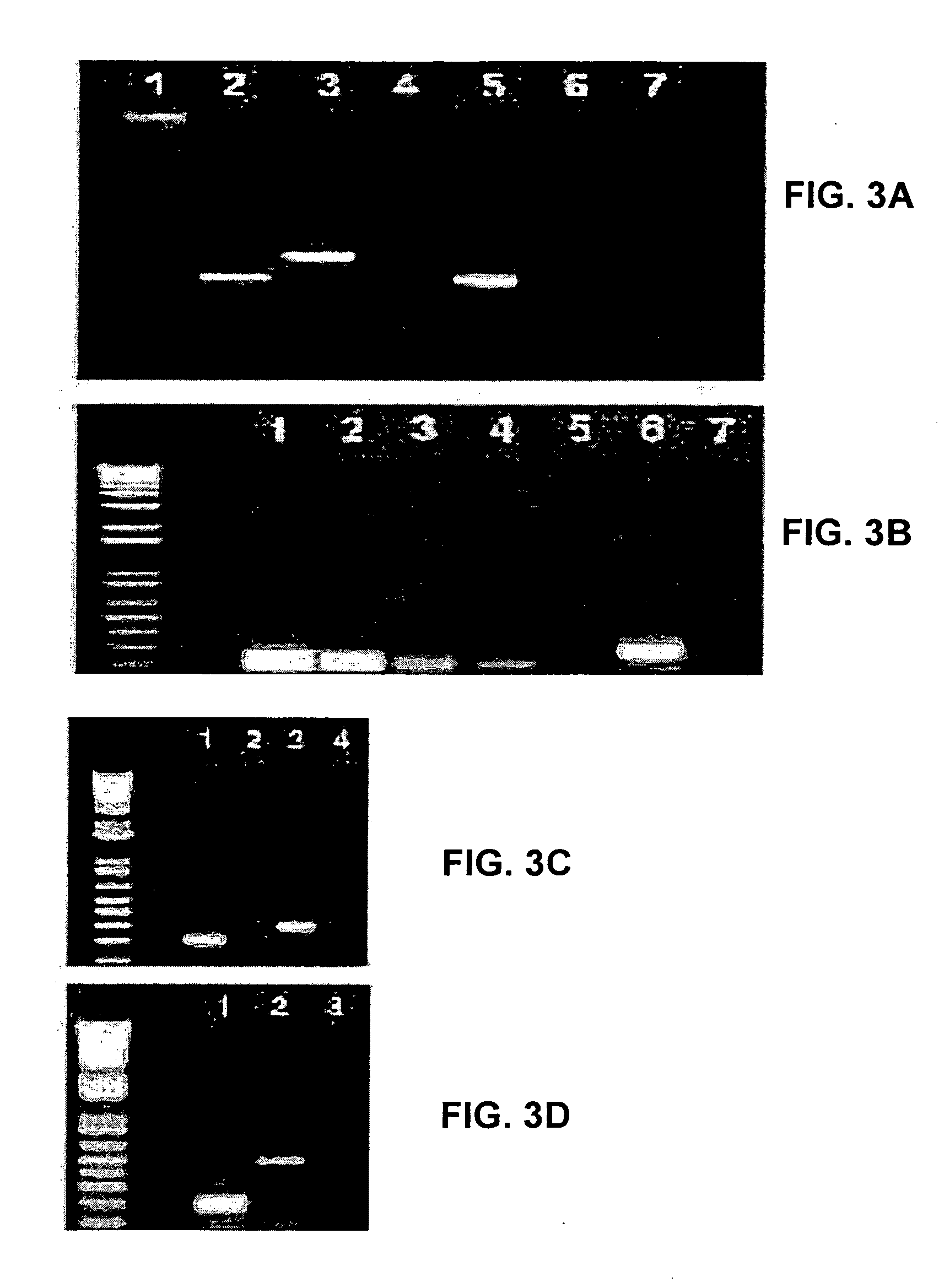
[0403] Both cell lines underwent spontaneous differentiation under standard culture conditions, but the process of spontaneous differentiation could be accelerated by suboptimal culture conditions. Cultivation to high density for extended periods (4-7 weeks) without replacement of a feeder layer promoted differentiation of human ES cells. In high density cultures, expression of the stem cell marker Oct-4 was either undetectable or strongly downregulated relative to the levels of the housekeeping gene beta actin (FIG. 3A-3B, lanes 5-7). Alphafetoprotein and human chorionic gonadotrophin were readily detected by immunoassay in the supernatants of cultures grown to high density. Alphafetoprotein is a characteristic product of endoderm cells and may reflect either extraembryonic or embryonic endodermal differentiation; the levels observed (1210-5806 ng / ml) are indicative of extensive endoderm present. Human chorionic gonadotrophin secretion is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Adhesivity | aaaaa | aaaaa |
| Responsivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com