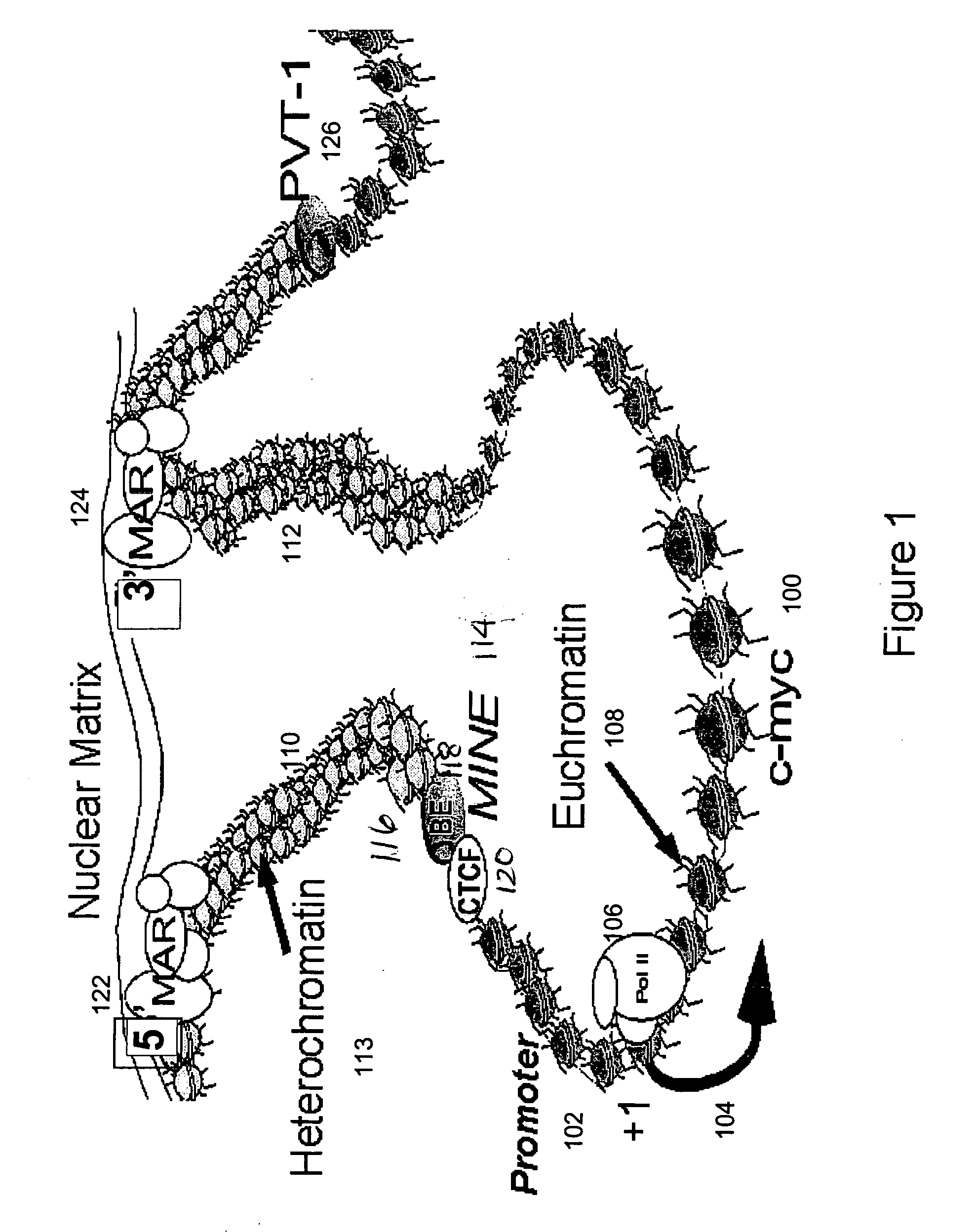
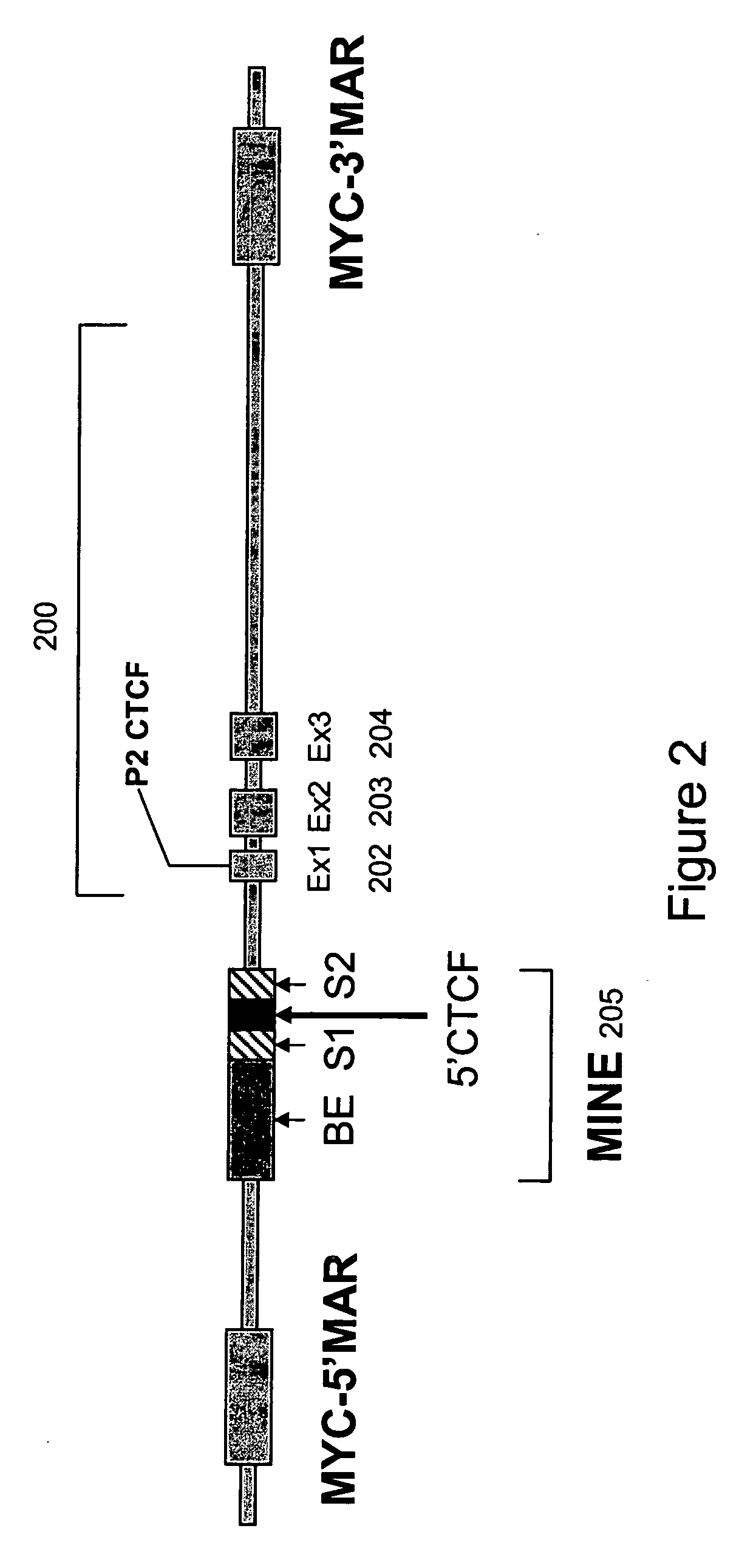
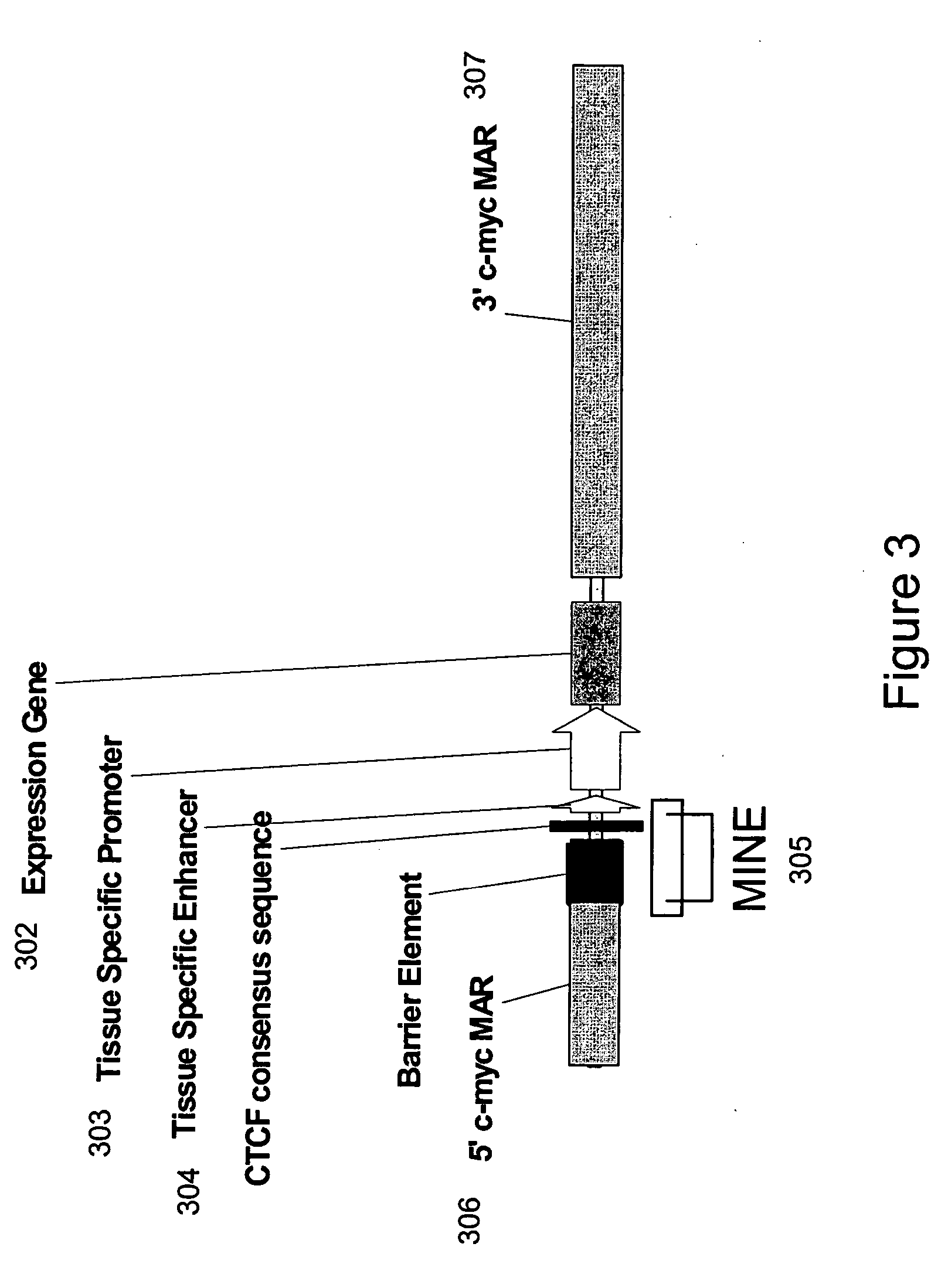
Recombinant vectors for use in position-independent transgene expression within chromatin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Boundary Definitions of Euchromatic and Heterochromatic Domains at the c-myc Locus
[0098] Transitions in the histone modification patterns mark the boundaries between euchromatic and heterochromatic domains at a gene locus [Litt et al., Embo J. 20: 2224-35 (2001)]. A high resolution map of histone acetylation and methylation patterns was generated within a 25 kb region of c-myc loci in both human and mouse (FIG. 5) by using a chromatin immunoprecipitation (ChIP) assay described below. CTLL2 cells, representing a murine T cell line, were exposed with interleukin 2 (IL-2) to induce c-myc transcription. HL60 cells of the human promyelocytic leukemia cell line were exposed with DMSO to induce differentiation, which correlates with c-myc expression downregulation. Chromatin immunoprecipitation assay (ChIP) was performed, involving the immunoprecipitation of formaldehyde-crosslinked chromatin from cell extracts using antibodies that specifically recognize modified forms of histone H3: (1)...
example 2
In Vivo Identification of Two CTCF-Binding Elements within 5′Boundary of c-myc
[0107] To determine the existence of functional binding sites for CTCF at the 5′boundary of the human and murine c-myc gene loci, in vivo associations between CTCF and regions of the 5′boundary were assayed by ChIP using a CTCF-specific antibody. Several potential CTCF-binding sites within the c-myc locus are listed in Table 2. The consensus sequence 5′-CCRNNAGRGG-3′ (SEQ ID NO:18) was previously characterized by Bell et al. [Cell 98: 387-96 (1999)]. Sites A (“P2”) (human SEQ ID NO: 20; mouse SEQ ID NO: 21) and B (“P1”) (SEQ ID NO: 19) were identified by in vitro binding studies [Fillipova et al., Nat. Genet. 28: 335-43 (2001)]. Alignment of CTCF sites positioned near the 5′boundary (human SEQ ID NO: 15; mouse SEQ ID NO: 16) located upstream of exon 1, and at the P2 promoter (human SEQ ID NO: 20; mouse SEQ ID NO: 21) located downstream of the beginning of exon 1, suggested a high level of conservation bet...
example 3
Functional Analysis of 1.6 kb c-Myc INsulator Element (MINE)
[0110] To determine the functional activity of the 1.6 kb MINE (SEQ ID NO:2) constituting the 5′boundary sequences of the human c-myc gene, it was placed at variable positions relative to enhancer and promoter elements within expression cassettes. FIG. 7 shows the positional effect of MINE in repressing or stimulating the transcription of a selectable marker reporter gene (neomycin), which was measured after its stable integration into genomic sites [Kellum and Schedl, Mol. Cell. Biol. 12: 2424-31 (1992)]. 5boundary elements were placed either between the enhancer and promoter, or upstream of the enhancer. The number of colonies growing under G418 selection was determined after a period of six weeks, by soft agar colony forming assay (method provided below), with the colony number being indicative of the number of cells that can express the neomycin selectable marker gene stably integrated into genomic sites. Jurkat cells ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Level | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com