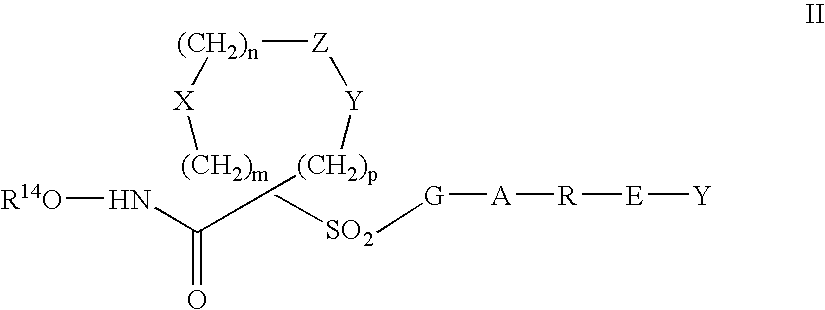
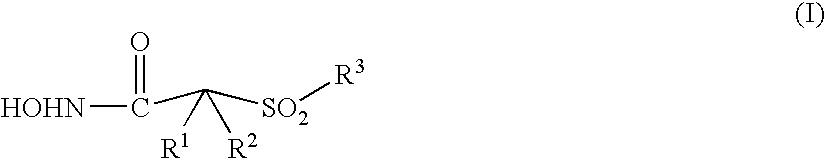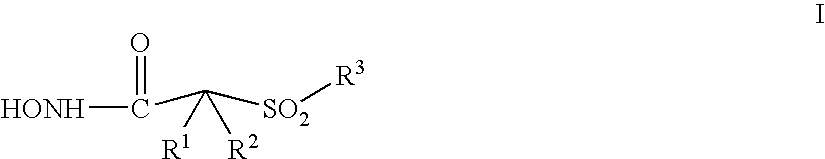Aromatic sulfone hydroxamic acid metalloprotease inhibitor
a technology of metalloprotease and aromatic sulfone, which is applied in the direction of amide active ingredients, drug compositions, cardiovascular disorders, etc., can solve the problems of low side effects, poor repair, and number of disease states, and achieve the effect of minimal side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of N-hydroxy-2-[(4-phenoxyphenyl)sulfonyl]acetamide
[0504]
[0505] Part A: To a solution of 3-bromopyruvic acid hydrate (1.95 g, 11.7 mmol) cooled to zero degrees Celsius in methanol (50 mL) was added 4-(phenoxy)benzenethiol (2.35 g, 11.7 mmol). The solution was stirred for 15 minutes followed by concentration in vacuo. The residue was partitioned between ethyl acetate and H2O and the organic layer was dried over magnesium sulfate. Concentration in vacuo provided the crude sulfide as a yellow solid that was used without any additional purification.
[0506] Part B: To a solution of the crude sulfide of part A (1.2 g, 2O cooled to zero degrees Celsius was added Oxone® (3.5 g, 5.72 mmol). The solution was stirred for 1 hour followed by removal of excess Oxone® by filtration. The filtrate was concentrated and the residue was dissolved into ethyl acetate and washed with saturated NaHCO3 and saturated NaCl and dried over magnesium sulfate. After concentration in vacuo the resulti...
example 2
Preparation of N-hydroxy-2-methyl-2-[(4-phenoxyphenyl)sulfonyl]propanamide
[0508]
[0509] Part A: To a solution of 4-(phenoxy)benzenethiol (3.8 g, 18.8 mmol) in methanol (60 mL) cooled to zero degrees Celsius was added t-butyl bromoacetate (2.8 mL, 18.8 mmol) and triethylamine (2.6 mL, 19.0 mmol). The solution was stirred for 30 minutes and was then concentrated in vacuo. The residue was partitioned between ethyl acetate and H2O and the organic layer was washed with saturated NaCl and dried over magnesium sulfate. Concentration in vacuo provided the sulfide as an oil. To a solution of the sulfide in dichloromethane (85 mL) was added m-chloroperbenzoic acid (13.8 g, 43.2 mmol) over 15 minutes. The solution was stirred at ambient temperature for 2 hours. The reaction was quenched by the addition of aqueous Na2SO3. After 30 minutes the solution was filtered through Celite®. The filtrate was washed with 25 percent aqueous hydroxylamine, 1N HCl, and saturated NaCl and dried over magnesium ...
example 3
Preparation of 1,1-dimethylethyl ester 4-[(hydroxyamino)carbonyl]-4-[(phenoxyphenyl)-sulfonyl]-1-piperidinecarboxylic acid
[0513]
[0514] Part A: A solution of 4-(phenoxy)benzenethiol (2.03 g, 10.0 mmol) in DMSO (DMSO; 20 mL) was heated to sixty-five degrees Celsius for 5 hours. The solution remained at ambient temperature for 18 hours. The solution was extracted with ethyl acetate and the combined organic layers were washed with H2O and saturated NaCl and dried over magnesium sulfate. Concentration in vacuo provided the disulfide as a yellow oil (2.3 g, quantitative yield).
[0515] Part B: To a solution of ethyl isonipecotate (15.7 g, 0.1 mol) in THF (100 mL) was added a solution of di-tert-butyl dicarbonate (21.8 g, 0.1 mol) in THF (5 mL) dropwise over 20 minutes. The solution was stirred overnight at ambient temperature and concentrated in vacuo to yield a light oil. The oil was filtered through silica gel (7:3 ethyl acetate / hexanes) and concentrated in vacuo to give the BOC-piperid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com