Method for preparing supported catalysts from metal loaded carbon nanotubes
a technology of carbon nanotubes and supported catalysts, which is applied in the preparation of metal/metal-oxide/metal-hydroxide catalysts, physical/chemical process catalysts, amino compound preparations, etc., can solve the problem of difficult to determine the exact chemical nature of the active catalyst component within the reaction zone, the cost of separating it from the reaction mixture, and the risk of contamination of the product. , to achieve the effect of improving structural integrity and structural integrity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0100] HNO3 oxidized CC carbon nanotube powders were created by pre-grinding HNO3 oxidized CC carbon nanotubes and sieved with a 20 mesh sieve. 70 ml of PdAc2 / acetone solution containing 0.148 g of PdAc2 was poured into a porcelain crucible with 7.0 g of HNO3 oxidized CC carbon nanotube powders to create a slurry, which was then stirred with a magnetic stirrer. After vaporizing most of the solvent at room temperature, the slush-like cake was dried under vacuum at 100° C. for 1-2 hrs.
[0101] The extrusion procedure was carried out with a Brabender device. (PLASTI-CORDER® 3 / 4” Laboratory Extruder. The screw has 25 flites and a compression ratio of 3:1). 14.0 g of deionized (“DI”) water were added to 6.0 g of 1 wt % Pd / nanotube powders at room temperature. The solid content in this dry-look mixture is around 30%. The mixture was extruded at room temperature and 30 RPM, and resulting extrudates were dried at 100-110° C. in a vacuum oven.
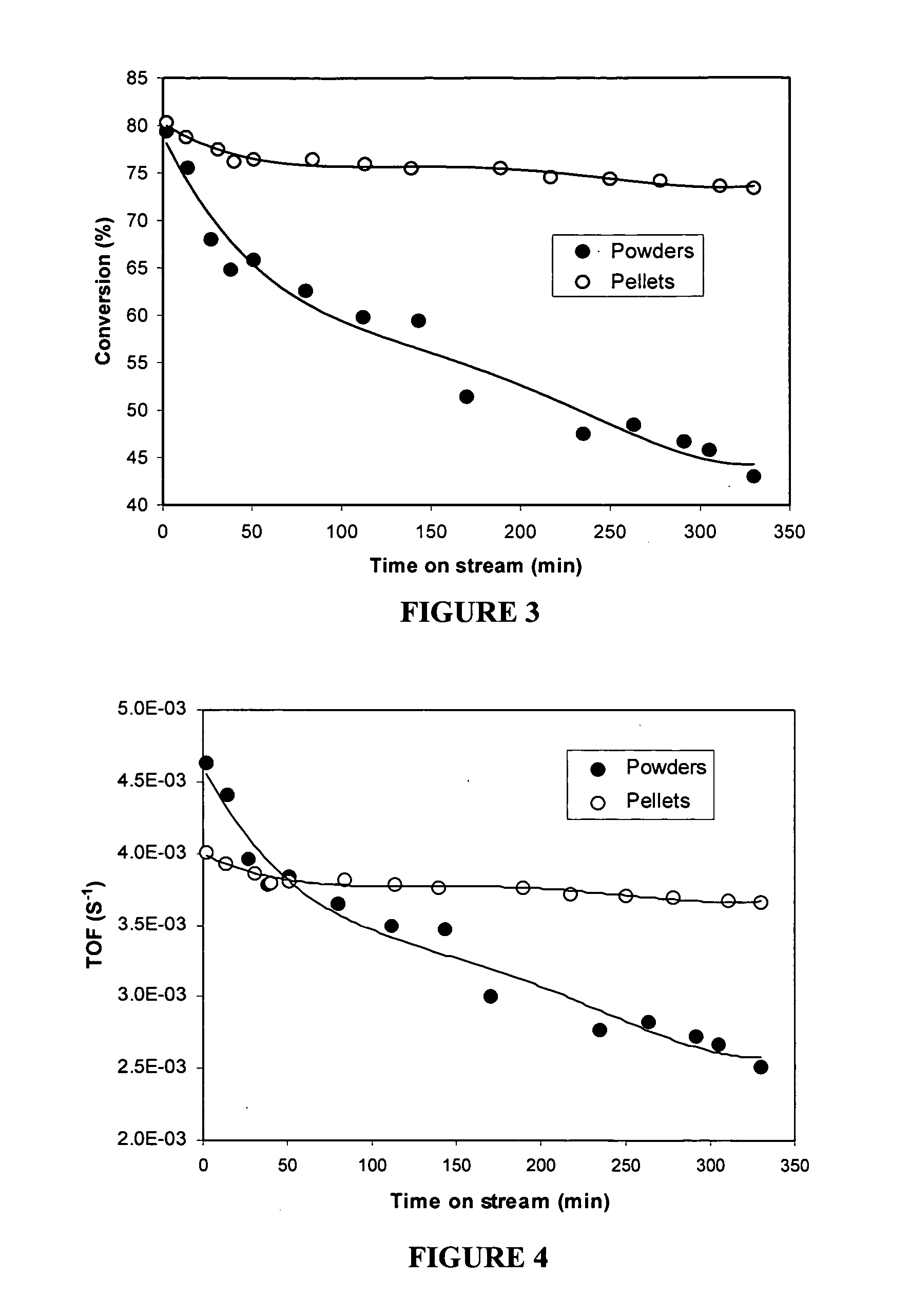
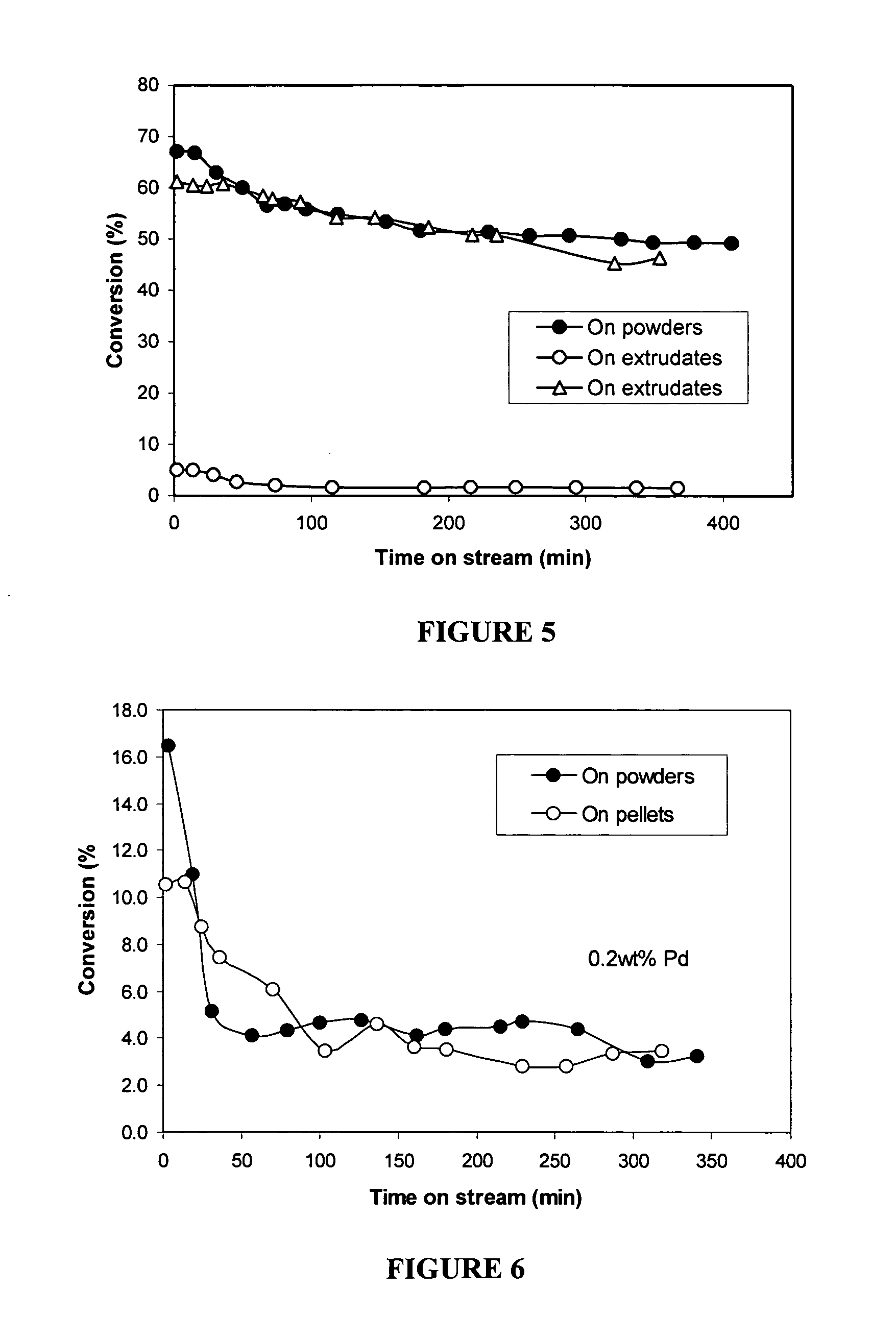
[0102] Two batches of extrudates were made from t...
example 2
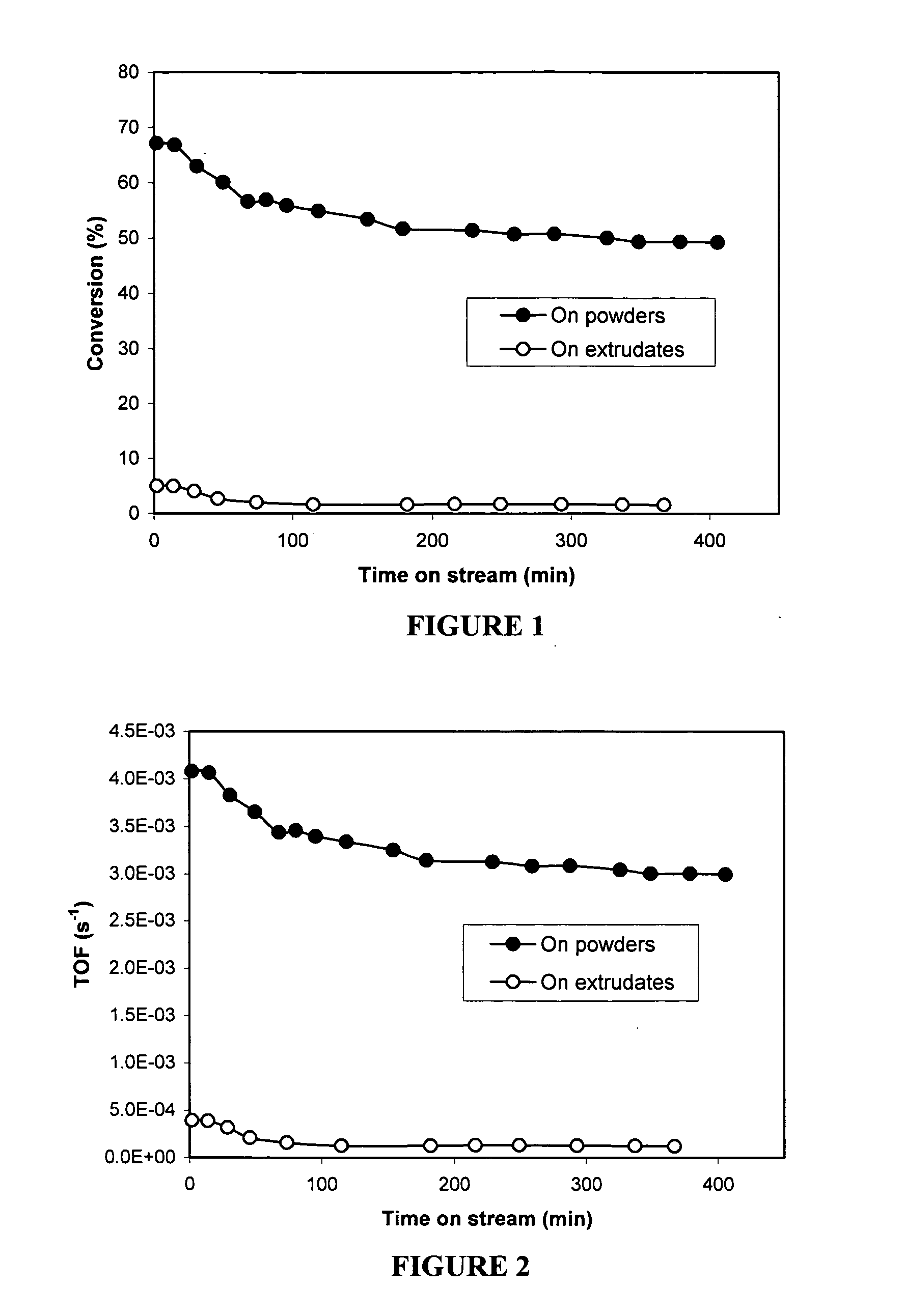
[0111] Comparison between the following two supported catalysts were made: (a) extrudates which have been loaded with Pd after extrusion vs. (b) CC nanotube powders which have been loaded with Pd and not extruded.
[0112] Extrudates were made from plain CC nanotubes with PAM-3K polymer binder, and calcined in Ar at 600° C. for 2 hrs. The extrudates were then oxidized with ozone in gas phase for 48 hrs at room temperature. The acid titer exhibited upon titration was about 0.968meq / g. Pd was loaded on the extrudates by ion exchange in Pd(NH3)4(NO3)2 solution at room temperature. The nominal loading of Pd is about 0.5 wt %.
[0113] Supported catalysts comprising Pd catalyst supported on powder CC nanotubes were made in a similar way. Namely, powder CC nanotubes were oxidized with ozone in gas phase for 48 hrs at room temperature. The acid titer exhibited upon titration was about 1.35 meq / g. Pd was loaded on the powder by ion exchange in Pd(NH3)4(NO3)2 solution at room temperature. The no...
example 3
[0117] Pd was loaded onto HNO3 oxidized CC nanotubes (i.e., CC aggregates which have been oxidized with HNO3) via ion exchange at room temperature in Pd(NH3)4(NO3)2 solution. The solution was evaporated and nanotubes with 0.5 wt % Pd supported thereon remained. The Pd / nanotubes were ground to powder. 0.6 g of H2O were added to 0.2 g of the Pd / nanotube powders. Half of the wet powder mixture was put into a ½″ pellet die. The die was pressured under 1,500 psi at room temperature for about 30 seconds. The thickness of the pellet is about 1.7 mm. The pellet was dried under vacuum at 100° C. for 3 hrs. The apparent Pd dispersions were measured by CO chemisorption for the Pd / nanotube powders (i.e., prior to die press) and the pellets (i.e., die pressed). The results are displayed in Table 5.
TABLE 5LoadingPd dispersionPd particleCatalyst(wt %)Form(%)size (nm)Pd0.5Powder50.02.2Pd0.5Pellet58.51.9
[0118] Table 5 revealed that the Pd / nanotube pellet has higher apparent Pd dispersion than the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface areas | aaaaa | aaaaa |
| pore diameters | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com