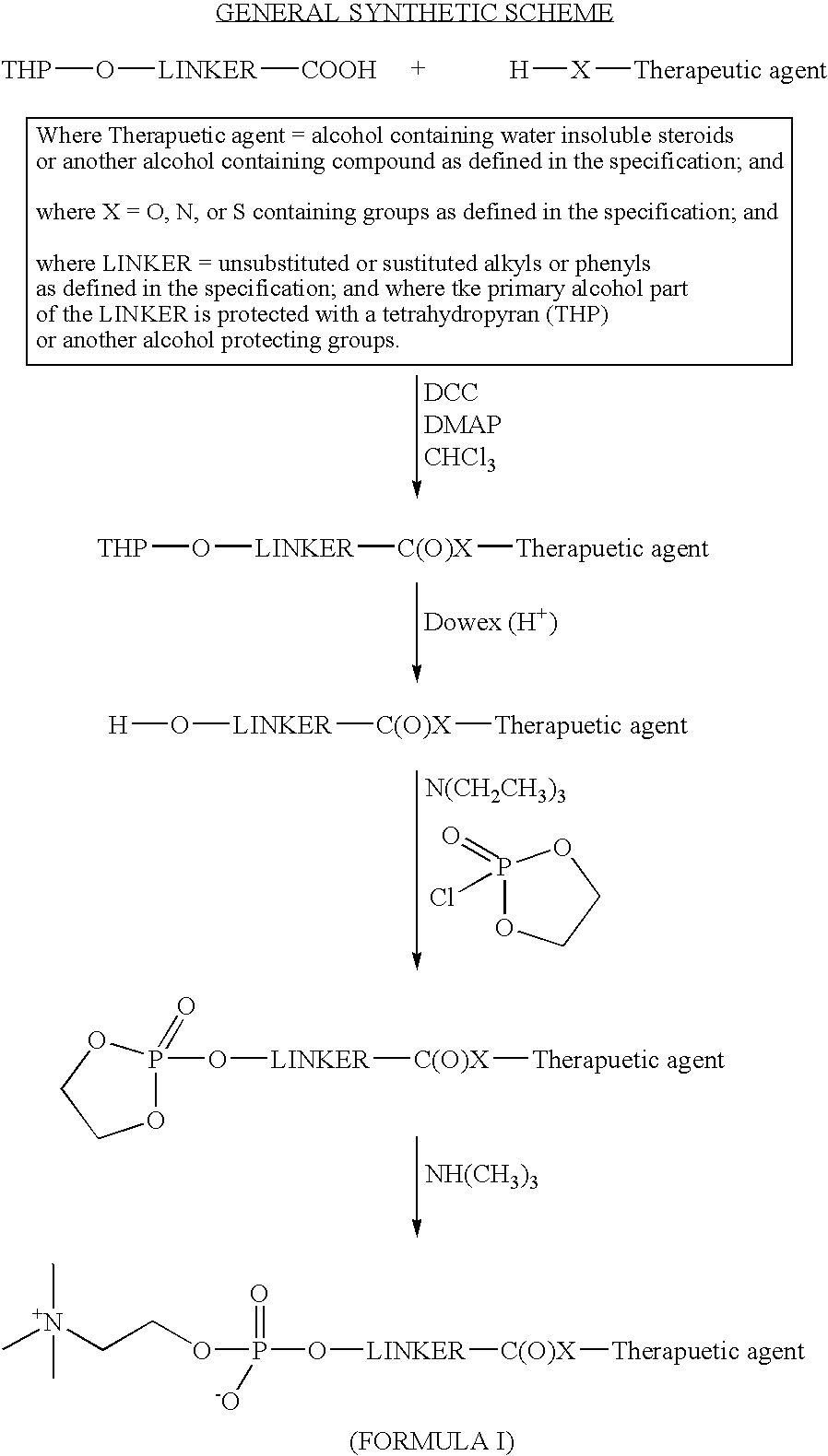
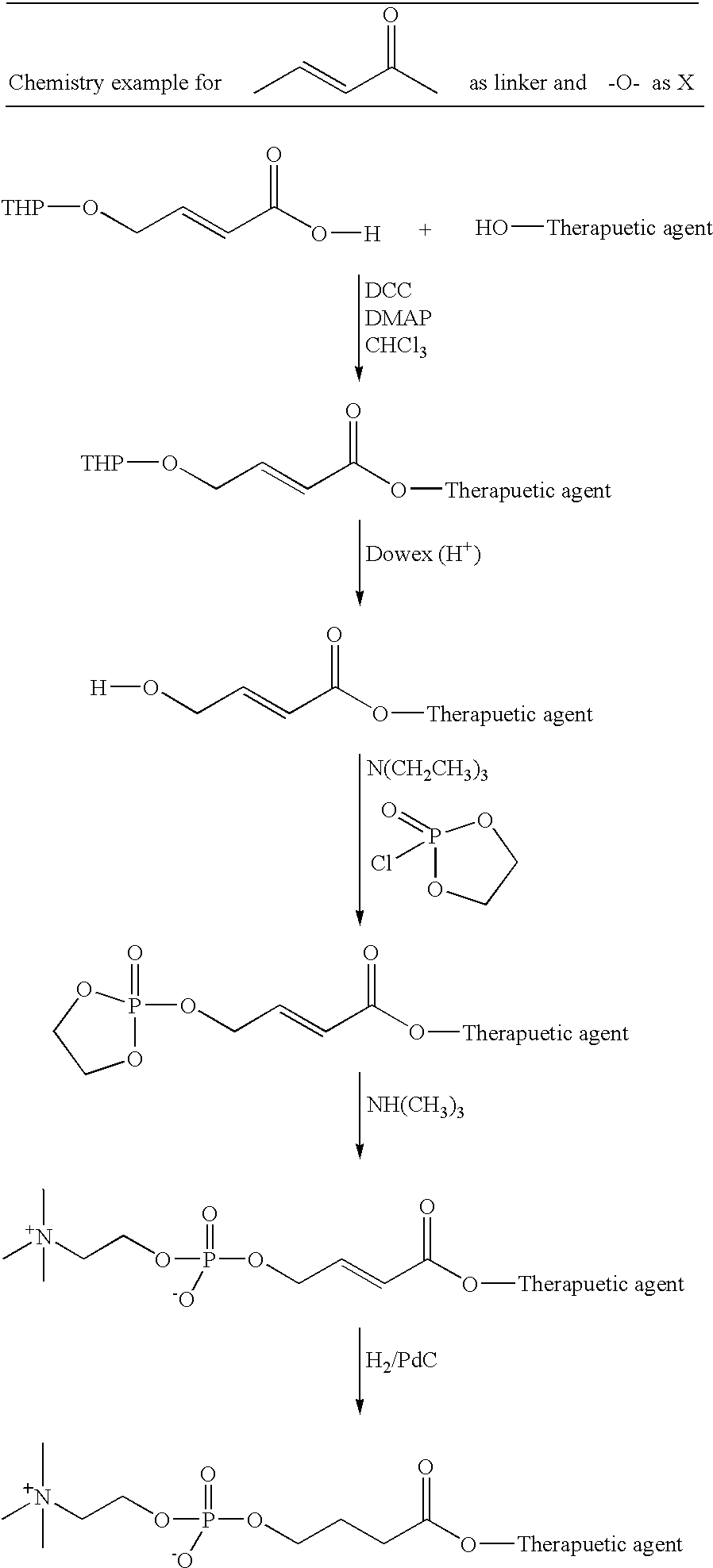
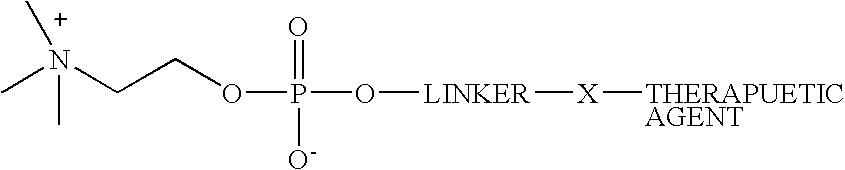Phosphocholine linked prodrug derivatives
a technology of phosphocholine and prodrug, applied in the direction of phosphorous compound active ingredients, drug compositions, biocide, etc., can solve the problems of inability to administer water insoluble therapeutic agents parenterally, hypotension, dyspnea, angioedema, etc., and achieve the effect of increasing the bioavailability of pharmaceutical agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Method A
Preparation of Phosphocholine-linked Propofol (Sedative / Anesthetic) {2′,6′-Diisopropylphenyl 4-(2-trimethyl ammonium ethyloxy)phosphonobutyrate}
[0090] Ethyl 4-hydroxycrotonate (trans) (Kende, Org. Syn. Col. Vol. VII, p 221) was treated with 2,3-dihydropyran and catalytic toluenesulfonic acid, according to Bernady (J. Org. Chem. 44, 1438, 1979) to yield ethyl 4-[2-tetrahydro pyranyl]oxycrotonate (trans). This compound was further treated with 0.1M LiOH in tetrahydrofuran, to yield the free acid (4-[2-tetrahydropyranyl] oxycrotonic acid), after acidification and work-up. This carboxylic acid was then coupled, via an ester bond, to 2,6-diisopropylphenol, utilizing N,N-dicyclohexyl-carbodiimide. After chromatographic purification on silica gel, the ester was then treated, in methanol, with a catalytic amount of Dowex 50W ion exchange resin to affect the removal of the tetrahydropyranyl protecting group. The resulting alcohol was treated with 2-chloro-2-oxo-1,3,2-dioxaphosphola...
example 2
Sleep Indication in Mice
[0092] The method which detects sedative activity following the protocol described by Simon et al. (J. Pharmacol. Paris, 13:241-252, 1982).
[0093] Mice (10 per group) are placed in Plexiglass cages (20×10×10 cm) and administered the test substance, propofol, produced as above as an i.v. bolus in two seconds. The latency to sleep and the occurrence of sedation / sleep are noted over a period of one hour. Sleep is indicated by the loss of the righting reflex. Animals within a group are tested sequentially and the test is performed blind. The test substance will be evaluated in 5 escalating doses. Unmodified propoful (16 mg per kg) administered in the same experimental conditions, will be used as a reference compound. The LD50 for hypnotic activity is calculated following the method of Lichtfield and Wilcoxin (J. Pharmacol. Exp. Ther. 96:99-113, 1949).
Lethal Dose 50 (LD50) in Mice
[0094] The method, which determines the acute dose of a test substance causing 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| water solubility | aaaaa | aaaaa |
| chemical | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com