Treatment of congestive heart failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0043] This example describes a study conducted to determine the effect of the treatment of the invention on endothelial function in Watanabe rabbits, known to develop complex atherosclerotic lesions during the first year of life. As previously mentioned, endothelial dysfunction is linked to the pathophysiology of CHF.
[0044] The rabbits entered the study at 7 to 8 months of age, and were randomized into three groups, a first group to be sacrificed immediately for baseline measurements, a second group (n=10) which received injections of blood treated according to the invention, and a third group (n=10) which received sham treatments comprising injections of untreated blood.
[0045] The treatment comprised a total of 4 injections of treated blood over a period of 10 weeks. The blood was treated by exposure to the following three stressors in an apparatus as generally described in U.S. Pat. No. 4,968,483 to Mueller et al.: [0046] (a) an elevated temperature of 42.5° C.±1.0° C.; [0047] ...
example2
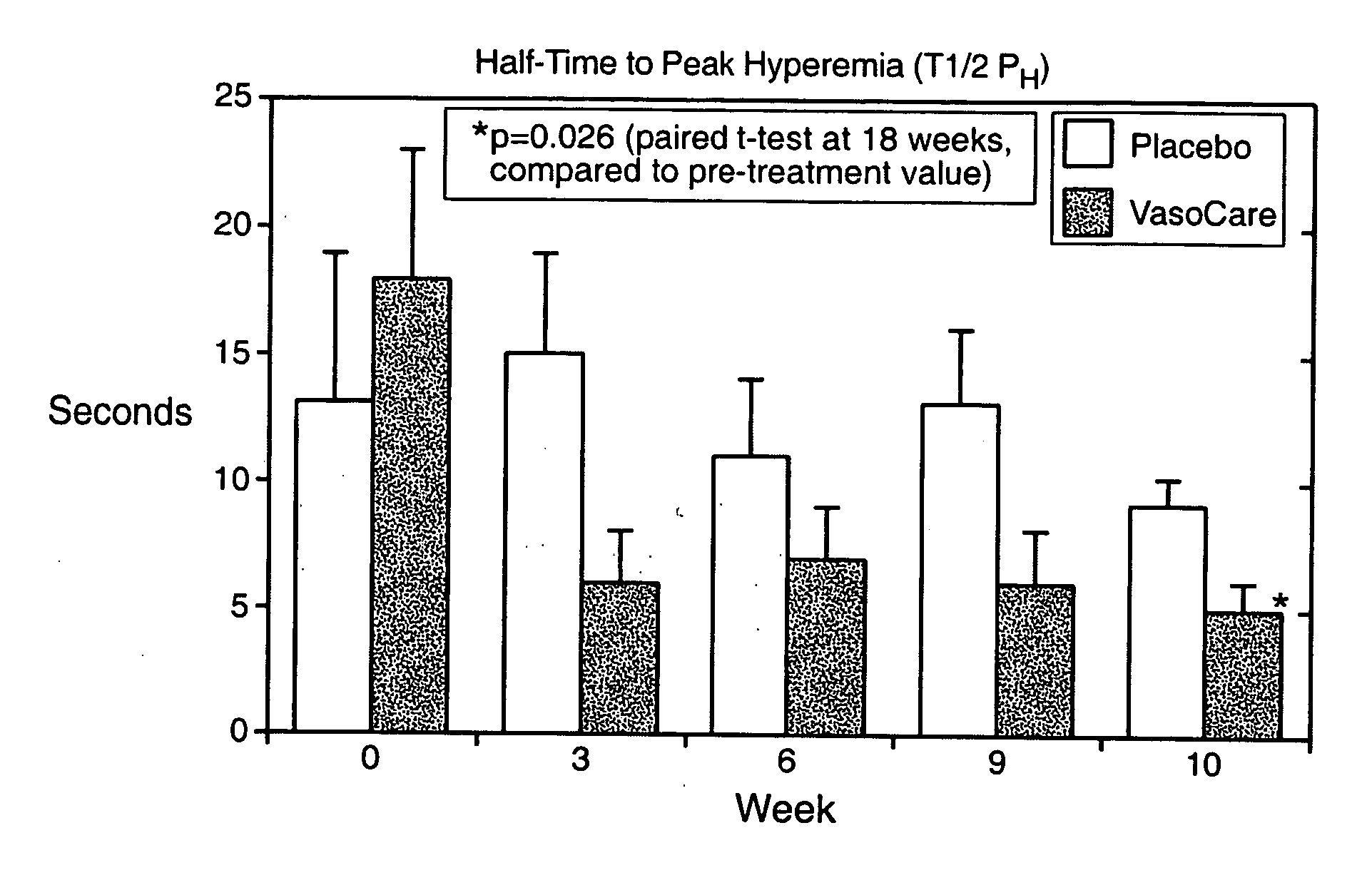
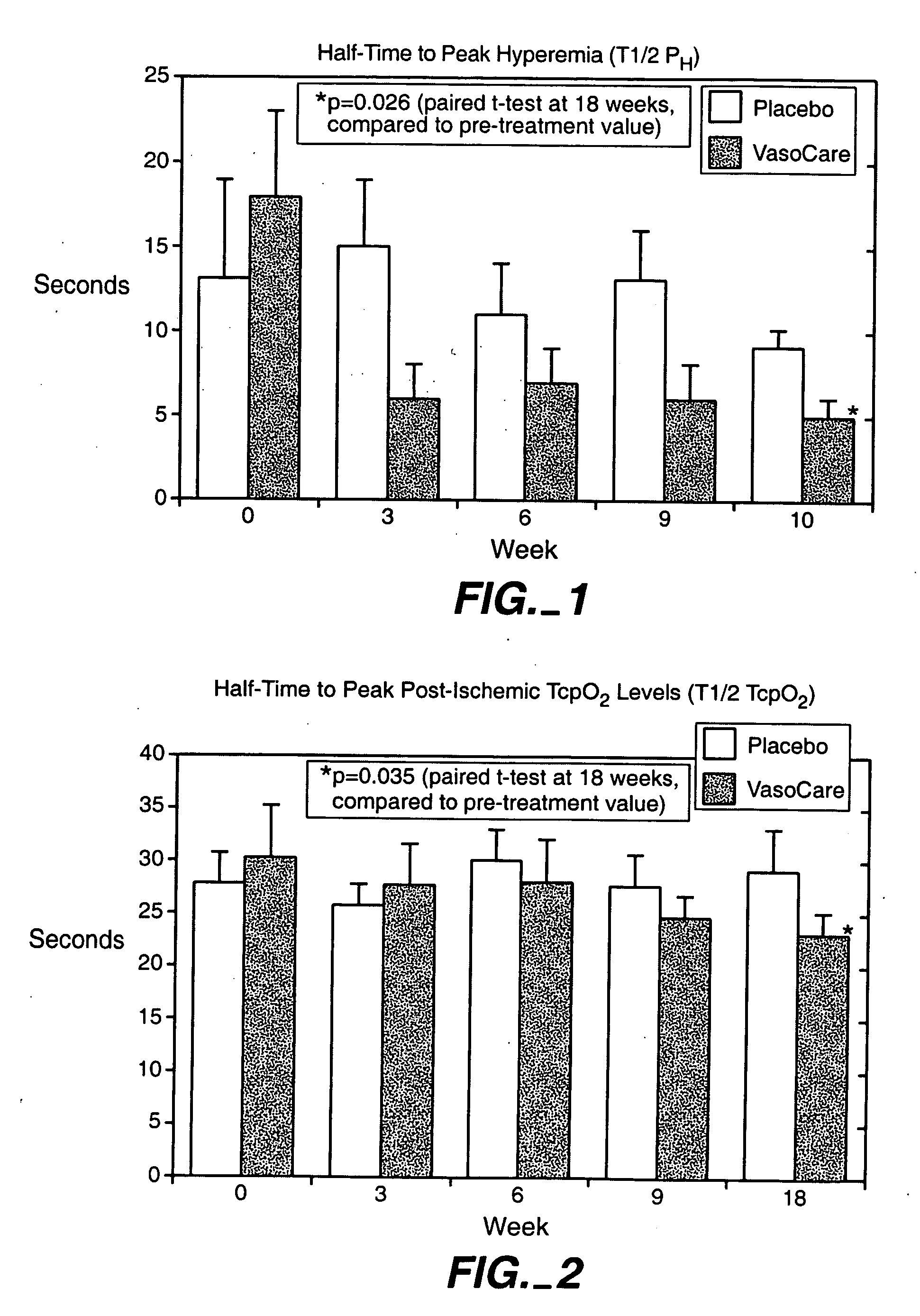
[0053] This example describes a study into the effects of the treatment of the invention therapy on patients suffering from peripheral vascular disease (PVD). The study was conducted at the University Hospital, Lund, Sweden.
[0054] The study comprised a placebo-controlled, double blind study in 18 patients (7 males, 11 females) with moderately advanced PVD, whose main symptom was intermittent claudication. The patients participating in the study were recruited from the attending population of the Department of Internal Medicine of the University Hospital, Lund, Sweden.
[0055] The patients were randomly assigned to receive either placebo (intramuscular injection of 10 ml warm saline) or treatment according to the invention comprising intramuscular injections of 10 ml of treated autologous blood. The treatment of the blood involved the collection of a 10 ml aliquot of a patient's venous blood into 2 ml of sodium citrate 3-4% as anticoagulant. Each blood aliquot was transferred to a st...
example 3
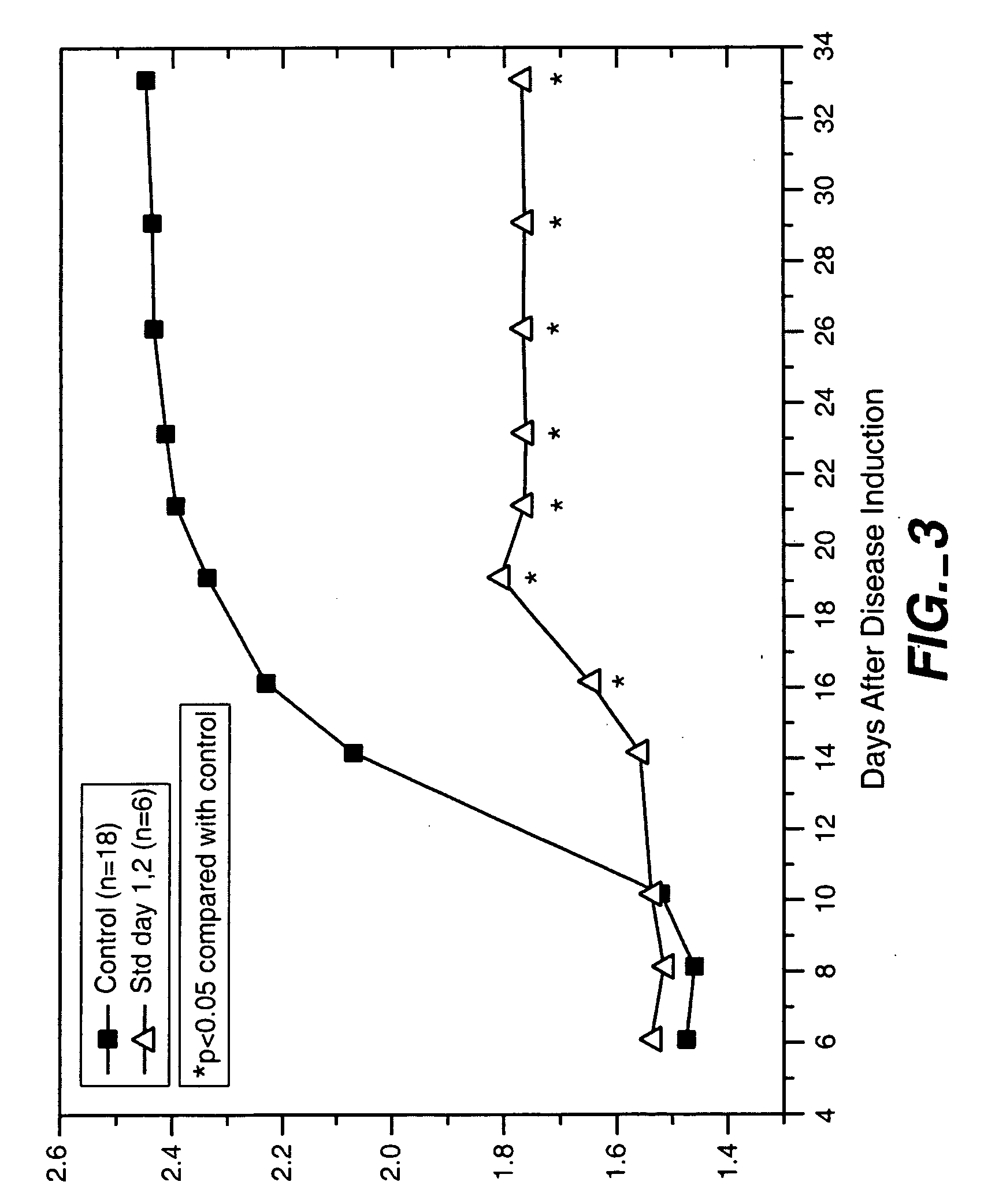
[0065] This example relates to the use of the treatment of the invention to prevent the onset of arthritis, and describes the results of a study conducted in an established animal model of arthritis. The specific animal model used in this study was adjuvant-induced arthritis in rats (see, for example, Pearson, C., 1956, “Development of Arthritis, periarthritis and periostitis in rats given adjuvant”, Proc. Soc. Exp. Biol. Med., 91:95). According to this model, arthritis is induced in rats by injecting them with adjuvant containing Mycobacterium butyricum.
[0066] Male Lewis rats, 4 to 5 weeks of age, 100 to 120 g, were obtained from Charles River Laboratories, quarantined one week and entered into the study. An adjuvant mixture was prepared for induction of arthritis by suspending 50 mg M. butyricum (Difco Laboratories, Inc., Detroit, Mich.) in 5 ml light white paraffin oil—m3516 (Sigma Chemical Co., St. Louis, Mo.) and thoroughly mixed using a homogenizer. Aliquots of the mixture su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com