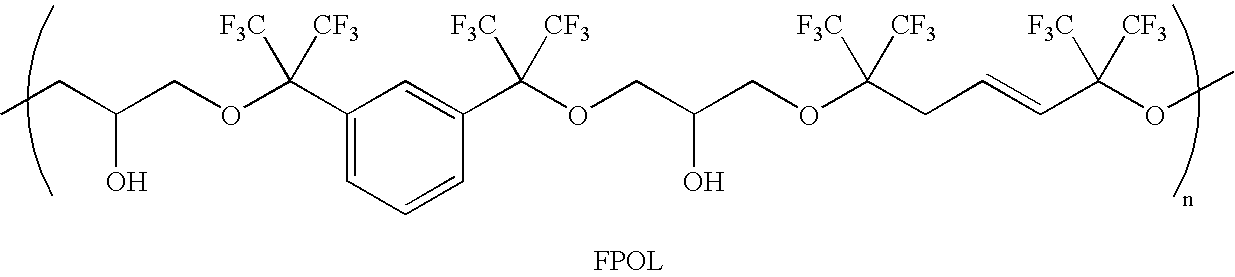
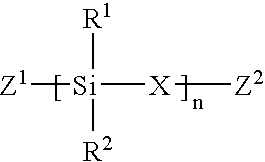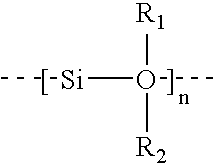Linear chemoselective carbosilane polymers and methods for use in analytical and purification applications
a technology of carbosilane and chemoselective, applied in the field of chemoselective polymer materials, can solve the problems of low density of functional groups, slow response and recovery of sensors, and weak acidity of hydrogen bonds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis Procedures
Preparation of Monomers
[0126] Allylbis(phenpropyl)silane: To a 500 mL Schlenk flask containing magnesium turnings (2.71 g, 111.5 mmol) in diethyl ether (250 mL) was added dropwise a solution of 1-bromo-3-phenylpropane (20.0 g, 100.5 mmol) over 4 hours. The resulting pale yellow solution was stirred for 10 hours at room temperature then cooled to 0° C. and treated with allyldichlorosilane (7.085 g, 50 mmol) via syringe. The resulting white slurry was stirred for 4 hours at room temperature then heated to reflux for 20 minutes. After filtration and aqueous work-up, the solvent was removed in vacuo to give a colorless liquid. Yield: 86%. FTIR (NaCl, cm−1): 3083, 3066, 3021, 2927, 2842, 2069, 1598, 1569, 1488, 1456, 1142, 1067, 1025, 987, 920, 891, 834, 745, 701. 1H NMR (CDCl3): 7.28, 7.17, 5.74, 4.89, 4.54, 3.74, 2.62, 1.67, 0.64.
[0127] Bis(phenpropyl)silane: To a 250 mL Schlenk flask containing magnesium turnings (0.70 g, 28.8 mmol) in diethyl ether (50 mL) was...
example 2
Applying a Thin Film to a SAW Device
[0134] SAW devices are cleaned in a Harrick plasma cleaner prior to polymer film application. Aerosol spray-coated films of the present invention in solvent are applied to a SAW device using an airbrush supplied with compressed dry nitrogen. The frequency change of the SAW device operating in an oscillator circuit is monitored during deposition, using the change in frequency as a measure of the amount of material applied. After application, the films are annealed at 50° C. overnight in an oven. Spray-coated films are examined by optical microscopy with a Nikon microscope using reflected light Nomarski differential interference contrast.
example 3
Detection of Basic Vapors with a Compound-Coated SAW Device
[0135] The polymers of the present invention are applied to SAW devices and tested against organic vapors at various concentrations. Upon exposure to a vapor, the coated acoustic wave devices undergo a shift in frequency that is proportional to the amount of vapor sorbed by the compound. Times to steady state response, corresponding to equilibrium partitioning of the vapor into the compound layer, are typically under 15 seconds using a vapor delivery system. From frequency shift data for a vapor at multiple concentrations, calibration curves are constructed. The calibration curves are nonlinear, which is consistent with hydrogen bonding interactions at a finite number of sites in the polymers of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass-to-rubber transition temperature | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com