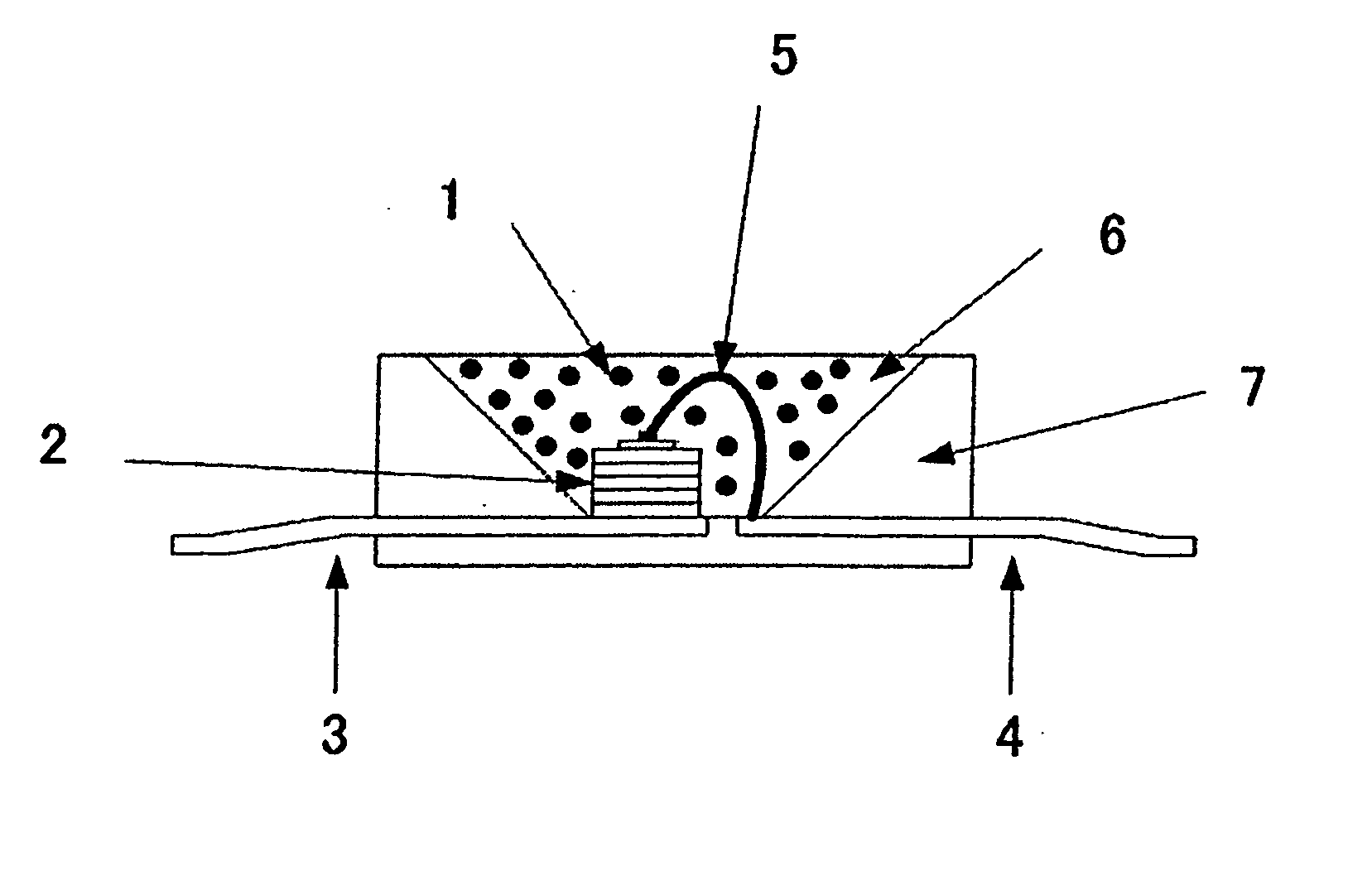
Oxynitride phosphor and light-emitting instrument
a technology of oxynitride and light-emitting instruments, which is applied in the direction of gas discharge electrodes, discharge tube/lamp details, solid-state devices, etc., can solve the problems of luminance drop, and achieve the effect of reducing material deterioration and luminance drop, and high luminan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0100] To obtain a compound represented by compositional formula La0.2Ce0.8Si5Al2O1.5N8.7 (Table 3 shows the mixing composition of the starting powders; Table 4 parameters; and Table 5 a post-reaction calculated composition), weighing was done such that silicon nitride powders having an average particle diameter of 0.5 μm, an oxygen content of 0.93% by weight and an α-type content of 92%, aluminum nitride powders, lanthanum oxide and cerium oxide were at 48.66% by weight, 17.06% by weight, 27.12% by weight and 7.16% by weight, respectively, and a 2-hour mixing was carried out in a wet ball mill using n-hexane.
[0101] n-Hexane was evaporated off in a rotary evaporator, and the ensuing mixture was molded in a mold at a pressure of 20 MPa into a compact of 12 mm in diameter and 5 mm in thickness.
[0102] That compact was placed in a boron nitride crucible, which was then set in an electric furnace of the graphite resistance-heating mode. Firing operation was started with evacuation of a...
examples 2-12
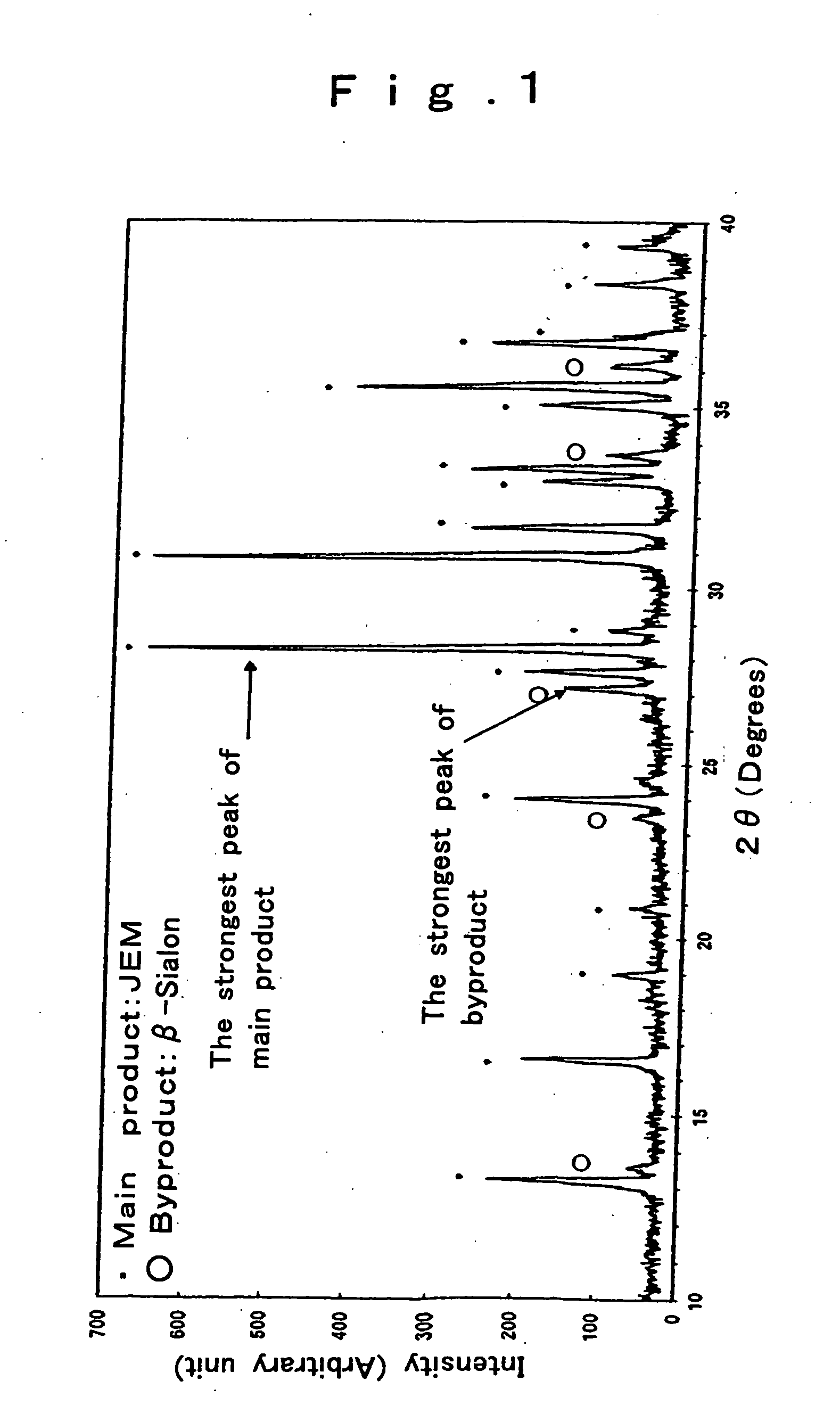
[0111] As in Example 1, oxynitride powders were prepared with the exception that the compositions shown in Tables 3, 4 and 5 were used. As a result, high-luminance fluorescent materials excited by ultraviolet radiation were obtained as set out in “Examples 2-12” in Table 6.
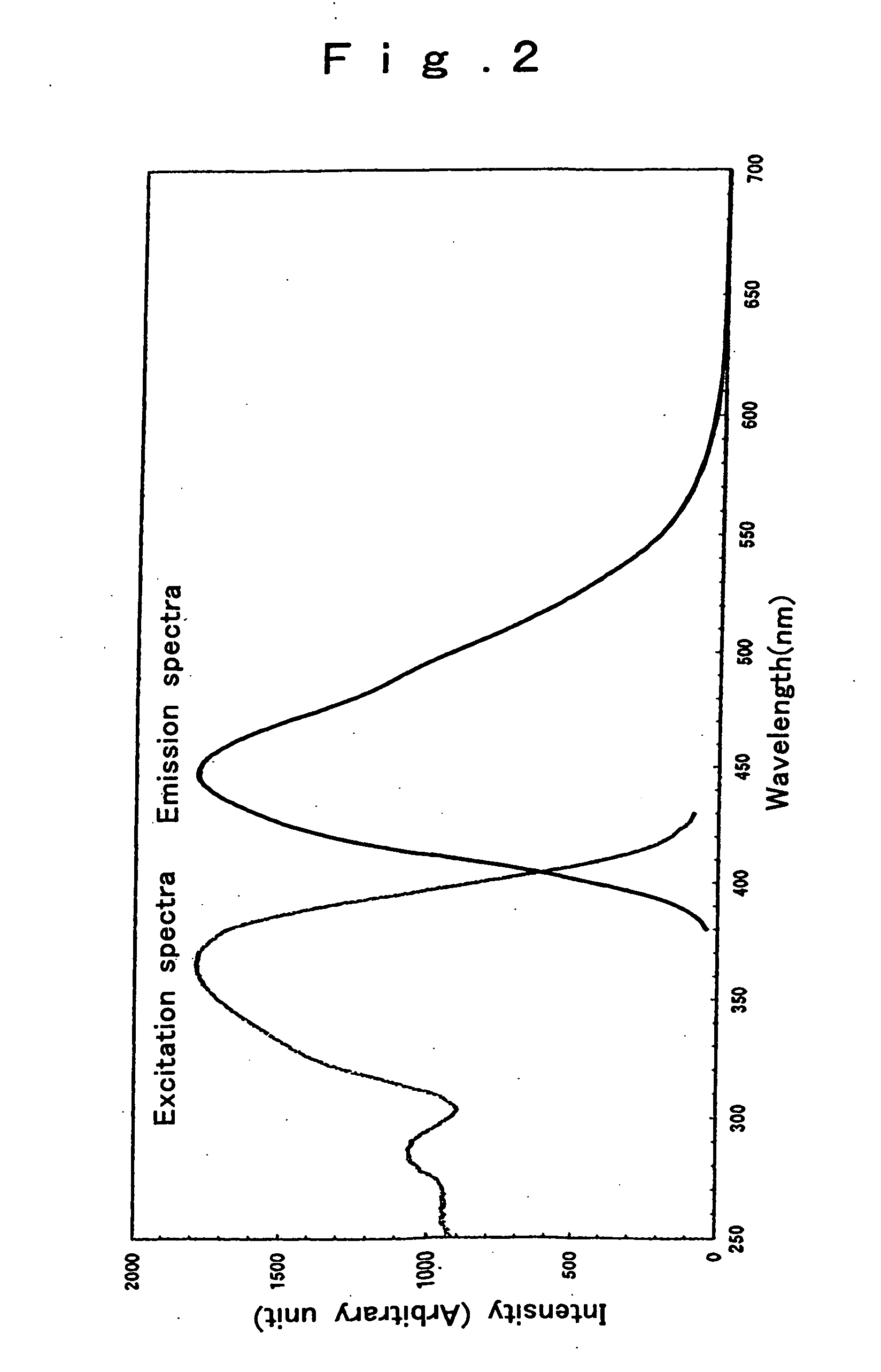
[0112] In Example 3 (Ce1Si5Al2O1.5N8.7), only Ce was contained as the rare earth element, and the obtained fluorescent material emitted 468-nm blue light, with spectra shown in FIG. 3. In Example 5 (La0.8Eu0.2Si5Al2O1.5N8.7), Eu worked as a luminescence center, and the obtained fluorescent material emitted green light of 510 to 550 nm with spectra shown in FIG. 4.
[0113] In Example 8 (La0.5Tb0.5Si5Al2O1.5N8.7), Tb worked as a luminescence center, and the obtained fluorescent material emitted green light with spectra shown in FIG. 5.
examples 14-32
[0118] The starting powders used herein were silicon nitride powders (having an average particle diameter of 0.5 μm, an oxygen content of 0.93% by weight and an α-type content of 92%), aluminum nitride powders (having a specific area of 3.3 m2 / g and an oxygen content of 0.79%), lanthanum oxide powders (with a 99.9% purity), europium oxide powders (with a 99.9% purity), aluminum oxide powders (with a 99.9% purity), lanthanum nitride powders obtained by synthesis by nitriding of metallic lanthanum (with a 99.9% purity) in ammonia, cerium nitride powders obtained by synthesis by nitriding of metallic cerium in ammonia, and europium nitride powders obtained by synthesis by nitriding of metallic europium in ammonia.
[0119] These starting powders were weighed in such a way as to have compositions as shown in Table 7. The composition was mixed in a mortar with an agate pestle for 30 minutes. Then, the resulting mixture was passed through a 500-μm sieve, and allowed to fall gravitationally ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| excitation wavelength | aaaaa | aaaaa |
| emission wavelength | aaaaa | aaaaa |
| excitation wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com