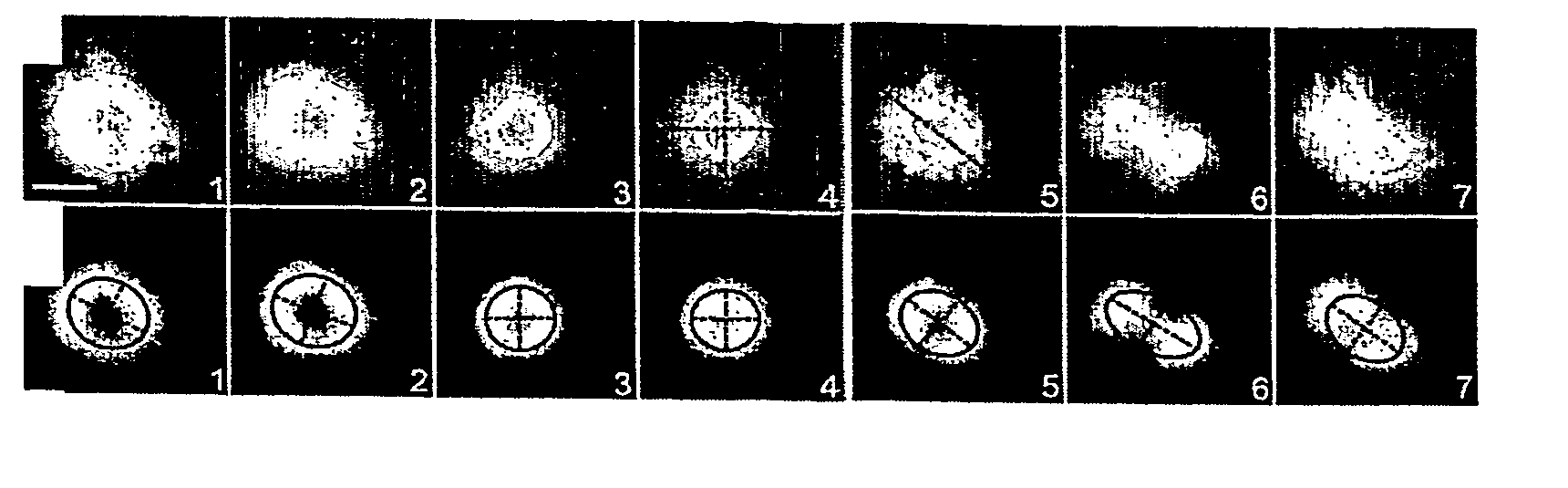
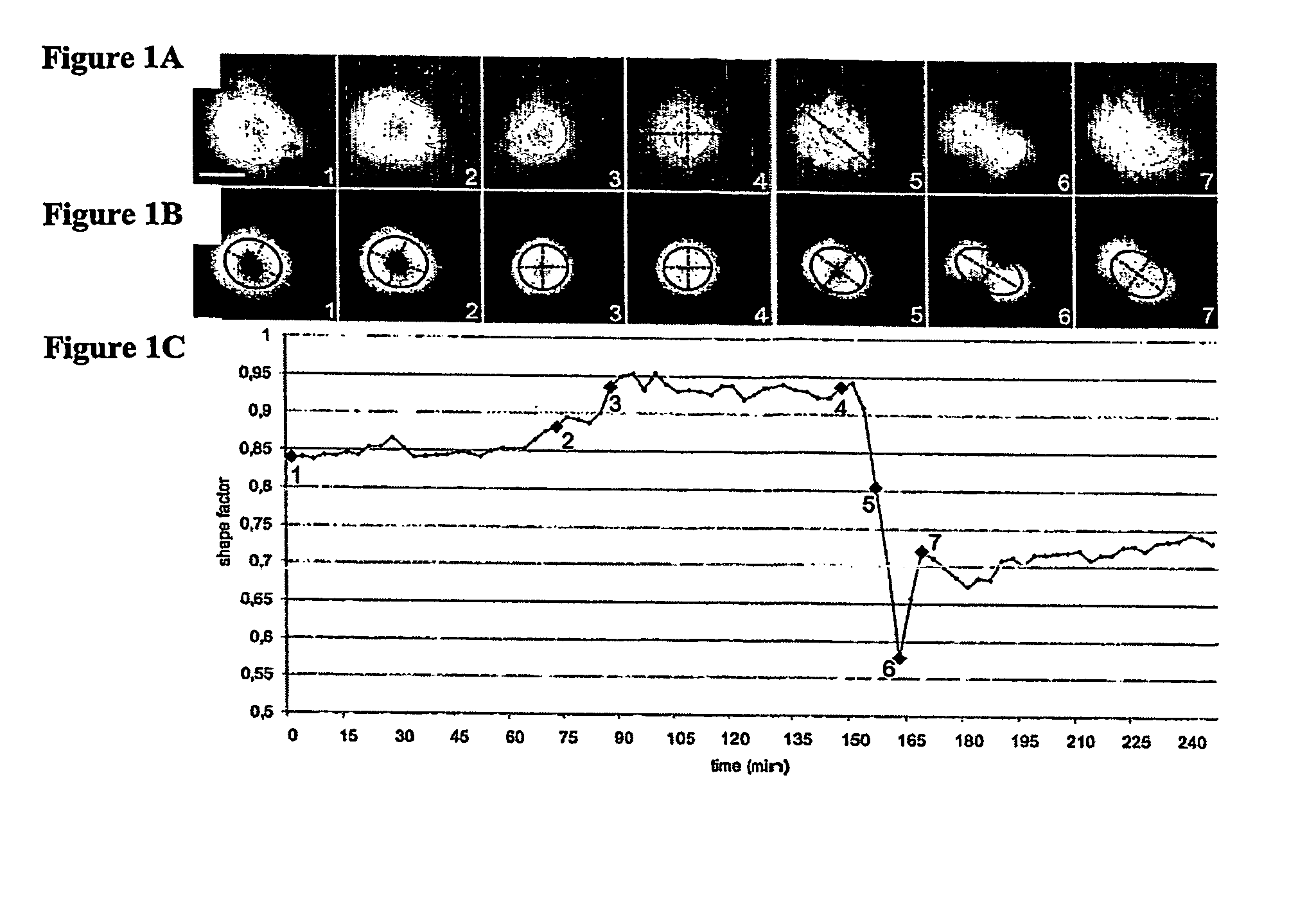
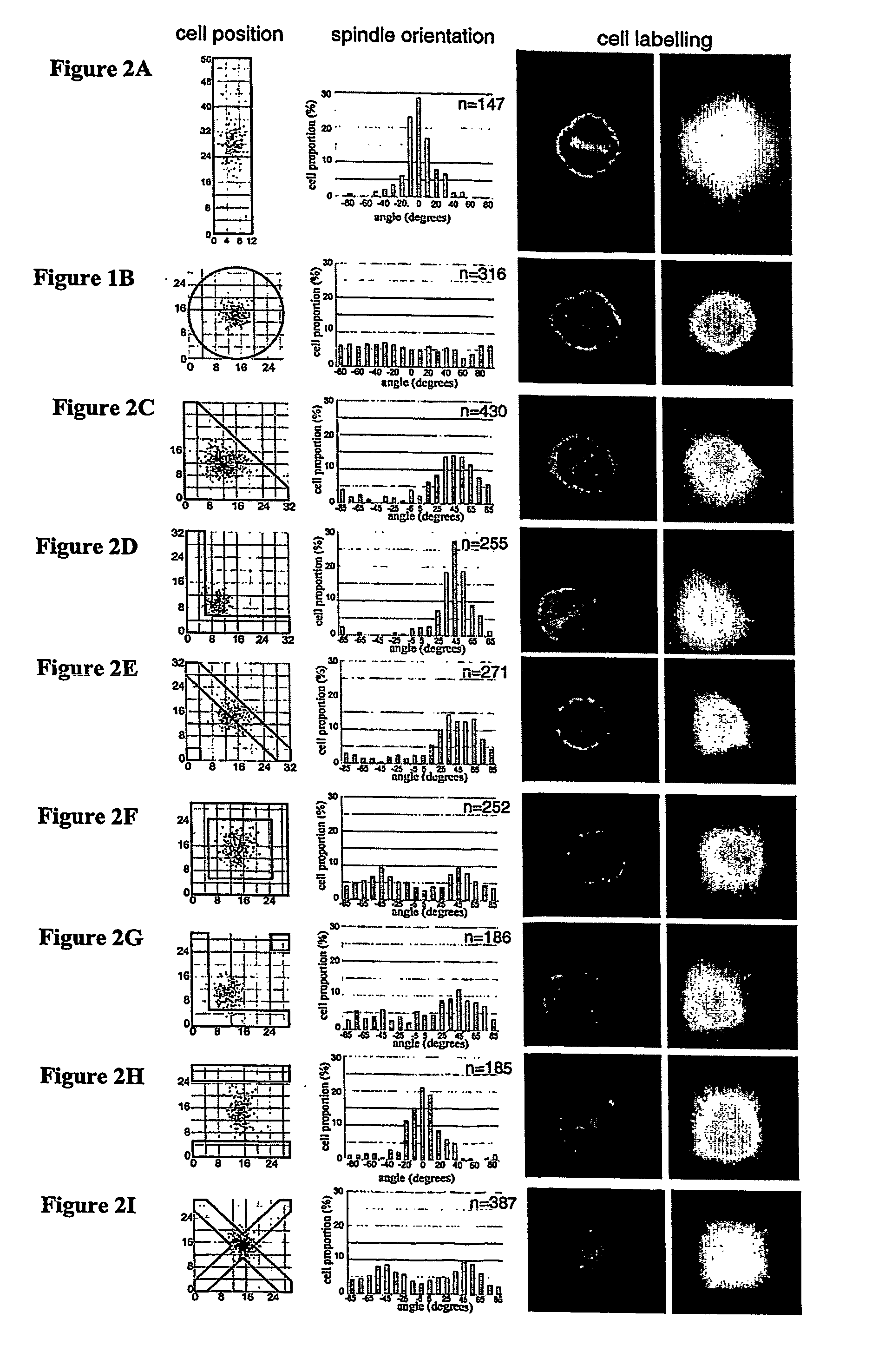
Methods and device for adhesive control of internal cell organisation
a technology of internal cell organization and adhesive control, which is applied in the direction of artificial cell constructs, instruments, enzymology, etc., can solve the problems of inability to use methods, inability to predict the distribution of the population on the bottom of the well, and cumbersomeness, so as to achieve accurate control of the intracellular distribution of each organelle, efficient and low cost, and precise control of focal adhesions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methods
Micropatterns Fabrication
[0177] Microcontact printing is a fully described method (Whitesides et al, Annu. Rev. Biomed. Eng., 2001, p 335-373). We made the poly-dimethyl siloxane (Sylgard, Dow Corning) stamps using a method described by Dr. A. Pépin (Pépin, A., Chen, Y., in Alternative lithography (ed. Sotomayor Torres C. M.) 305-330, Boston / Dordrecht / London, 2003). The glass coverslip treatment we used has been developed by Dr P. Nassoy (Cuvelier et al. Eur. Biophys. J. 32, 342-354 (2003). A stamp was inked with a 50 μg / mL fibronectin solution, 10% of which was labelled with Cy3 (Amersham Biosciences), for 5 minutes, dried and placed in contact with a silanised coverslip for 5 minutes. After removal of the stamp, the printed coverslip was immersed in PBS containing 20 mg / mL maleimide-poly(ethyleneglycol), PEG-mal (Nektar Therapeutics) for 1 hour at room temperature. The coverslip was then gently washed in PBS ready for cell deposition.
Cell Culture and Deposition
[0178]...
example 2
Materials and Methods
Chemicals
[0198] PDMS,: Sylgard, Dow Corning, ref: 1673921, 184 silicone elastomer kit.
[0199] Mercapto-silane: Roth Sochiel, ref: SIM6476.0, 3 mercapto-propyulrimethoxy silane.
[0200] PEG-mal: Shear Water mPEG-MAL MW: 5000, ref 2D2MOH01.
[0201] Fibronectin: Sigma
[0202] Trypsin
[0203] A master template has been made using a photolithographic method developed and fully described by Whitesides et al (Annu. Rev. Biomed. Eng., 2001, p 335-373). Briefly, a silicon wafer coated with a photoresist layer was illuminated with UV through a chrome mask on which the pattern has been designed with an electron beam. An elastomeric poly(dimethylsiloxane), PDMS, stamp was casted on the master and cured 3 hours at 60° C. to reproduce the negative features of the master. The stamp of PDMS was sonicated in ethanol 70% for 2 minutes and dried with blowed air, then oxidized in an air plasma for 10 s and inked with the protein solution, 50 μg / mL of fib...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle | aaaaa | aaaaa |
| time- | aaaaa | aaaaa |
| time- | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com