Dibenzosilol Polymers, Their Preparation and Uses
a technology of dibenzosilol and polymer, applied in the field of dibenzosilol, can solve the problems of less electrochemical stability prone to degradation, and shorter life of materials containing these lower electron affinities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
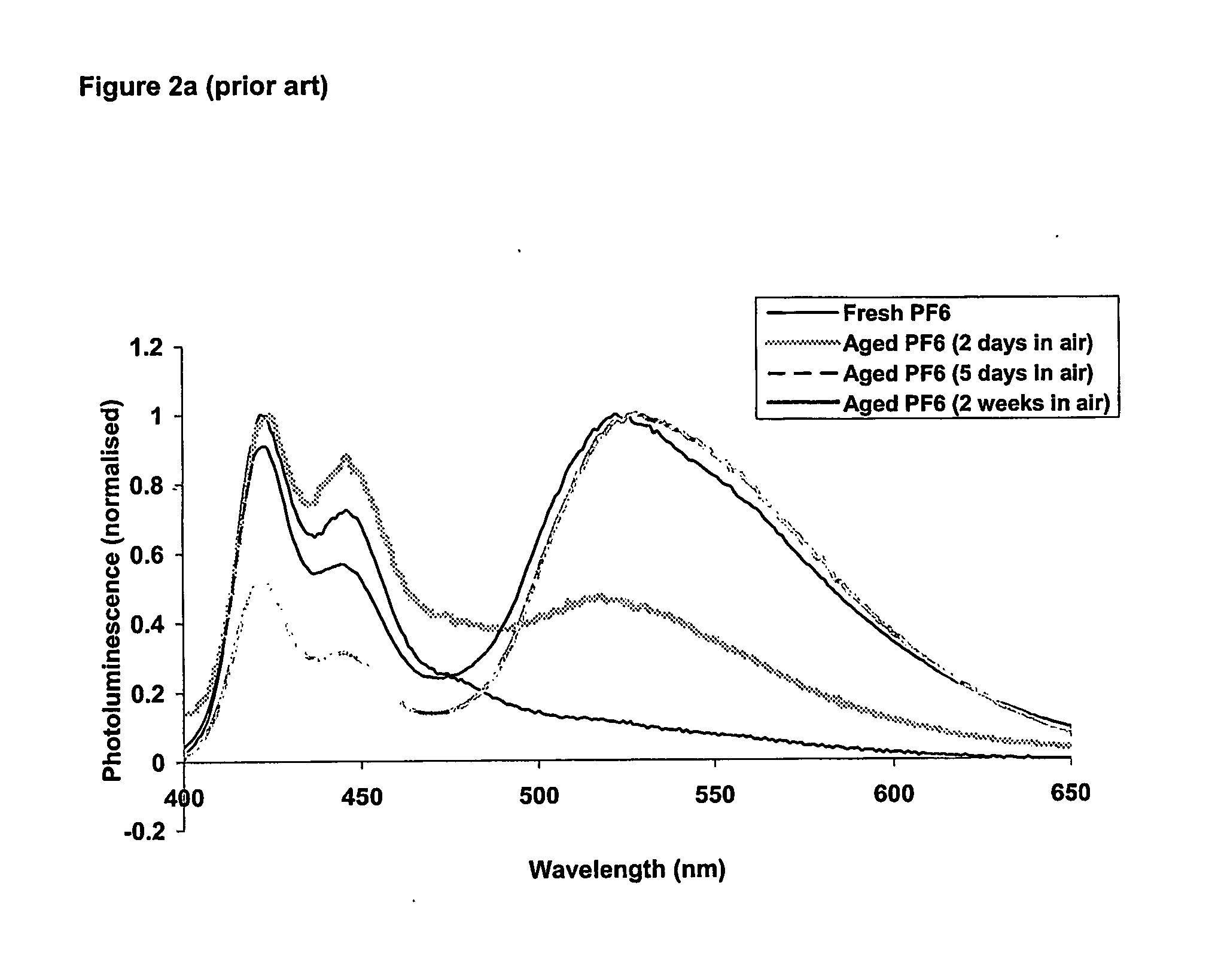
Image
Examples
polymer example 1
[0130] A homopolymer according to the first aspect of the invention was prepared by Suzuki polymerisation of Monomer 1 and Monomer 2 followed by end-capping with bromobenzene and phenylboronic acid according to the following scheme to afford dibenzosilole polymer PS6:
[0131] To a dried Schlenk tube was added 2,7-dibromo-9,9-dihexyl-9H-9-dibenzosilole (84 mg, 0.17 mmol, 1.0 equiv.), 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)-9H,9-dihexyldibenzosilole (100 mg, 0.17 mmol, 1.0 equiv.), palladium(II) acetate (1.0 mg, 45 μmol, 2.7%) and tricyclohexylphosphine (5 mg, 178 μmol, 10.7%) under nitrogen atmosphere. Dry toluene (2.5 cm3) was added and the mixture was stirred at 90° C. for 5 min. 20% w / w Tetraethylammonium hydroxide aqueous solution (1.0 cm3) was then added. The mixture was stirred for a further 2 h. To the mixture was then added phenylboronic acid (20.3 mg, 17 mmol, 1.0 equiv.), and after stirring for 1 h, bromobenzene (26.1 mg, 17 mmol, 1.0 equiv.) was added. After ...
polymer example 2
[0132] A copolymer according to the invention was prepared by Suzuki polymerisation as disclosed in WO 00 / 53656 with a diboronic acid of di(n-hexyl)fluorene followed by end-capping with bromobenzene and phenyl boronic acid to afford Polymer PS6F6 as shown below:
Poly(9,9-dihexyl-2,7-fluorenyl-alt-9,9-dihexyl-2,7-silafluorenyl)
[0133]
[0134] To a dried Schlenk tube was added 9,9-dihexyl-2,7-dibromo-9H-9-dibenzosilole (84 mg, 0.17 mmol, 1.0 equiv.), 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)-9,9-dihexylfluorene (97 mg, 0.17 mmol, 1.0 equiv.), palladium(II) acetate (1.0 mg, 45 μmol, 2.7%) and tricyclohexylphosphine (5 mg, 178 μmol, 10.7%) under nitrogen atmosphere. Dry toluene (2.5 cm3) was added and the mixture was strred at 90° C. for 5 min. 20% w / w Tetraethylammonium hydroxide aqueous solution (1.0 cm3) was then added. After 1 hour, the mixture became very viscous and additional dry toluene (1.0 cm3) was added. The mixture was stirred for a further 1 h. To the mixture was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phosphorescence quantum yield | aaaaa | aaaaa |
| Electronegativity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com