Method for the gasification of moisture-containing hydrocarbon feedstocks
a technology of hydrocarbon feedstocks and hydrocarbon feedstocks, which is applied in the direction of molten salt/metal gasification, combustible gas catalytic treatment, combustible gas production, etc., can solve the problems of high capital cost of coal gasification plants, prohibitively high gas cost, and too low for manufacturing high-value hydrogen-containing materials that are needed for commerce, etc., to achieve a higher co:co2 ratio and reduce capital cost , the effect o
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
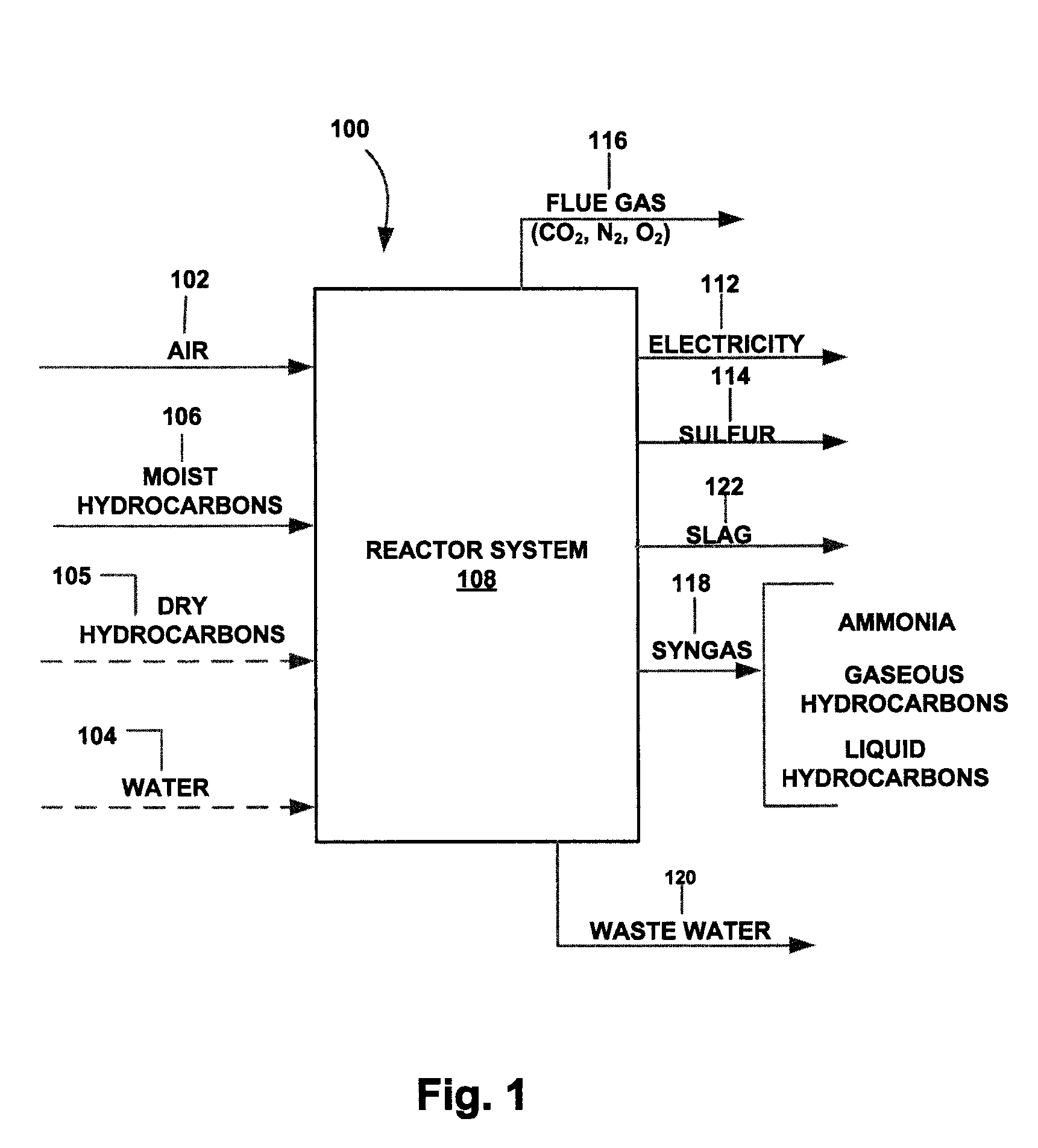
[0072] An overview of the method of the present invention is illustrated in FIG. 1. The method 100 includes providing reactants to a reactor system 108, where the reactants include at least air 102 and a moist hydrocarbon feedstock 106 that comprises H2O in appreciable quantities.
[0073] According to the present invention, the hydrocarbon feedstock 106 can advantageously include relatively low-value hydrocarbons, including those having less than about 10 mol. % H2 and which can also include impurities such as sulfur.
[0074] More specifically, the hydrocarbon feedstock according to the present invention can include low-cost, high heating-value carbon sources such as petroleum coke, scrap tires and liquid petroleum residues; medium cost, high heating-value and low-ash, high rank coals that may contain high levels of sulfur; or low-cost, low-heating value materials such as low-rank (sub bituminous) coal, biomass and the organic portion of municipal waste products. Plastics contained in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com