[0031] In one embodiment, the invention employs an acceleration sensor to characterize displacement and vibrational LV motion, and uses this motion data to characterize the different phases of the LV cycle for analyzing LV function. In another embodiment, the invention measures acceleration in at least two different frequencies with either two or more sensors or two or more frequency filters to characterize LV motion. In yet another embodiment, the invention senses
high frequency (greater than about 150 Hz) low amplitude motion related to valvular
pathology (e.g., mitral regurgitation),
mid frequency (between about 20 Hz and 150 Hz) lower amplitude motion related to isovolumic contraction / relaxation and valve closure, and
low frequency (less than about 20 Hz)
high amplitude motion signals related to displacement of the LV occurring during the ejection phase and early and late
diastole. In still a further embodiment, the invention identifies a target pacing region or regions in the LV or RV using an acceleration sensor by localizing regions of
late onset of motion relative to the QRS, or isovolumic contraction, or
mitral valve closure, or by pacing of target regions and measuring LV function in response to pacing. In another embodiment, the invention measures myocardial motion with an
accelerometer relative to the onset of isovolumic relaxation or
aortic valve closure to determine contractile reserve and / or the presence of post-systolic shortening. In yet another embodiment, the invention identifies target pacing regions in the LV using an acceleration sensor by pacing different regions and measuring the regional and / or global LV
functional response to pacing. In still a further embodiment, the invention uses an acceleration sensor to measure LV function by sensing changes in the time interval length of the LV
cardiac cycle phases (isovolumic contraction / relaxation, ejection, and / or filling); changes in mitral regurgitation
signal amplitude and duration; and changes in peak amplitude and slope of isovolumic contraction, isovolumic relaxation, and ejection phases; and frequency changes of the isovolumic contraction and relaxation phases. In another embodiment, the invention may use an acceleration sensor to identify patients with LV dyssynchrony or asynchrony and a normal QRS width (90-120 ms), a modestly increased QRS width (120 to 150 ms), and wide QRS or LBBB pattern (QRS>150 ms). In yet another embodiment, the invention facilitates identification of the coronary
ostium and LV
vein branches into the
coronary sinus for cannulation with guidewires or catheters. In a still further embodiment, the invention provides the physician with data from acceleration sensing for management of optimal pharmacologic treatment. In yet another embodiment, the invention provides a
wireless acceleration sensing
medical device and
system for assessing LV motion and function.
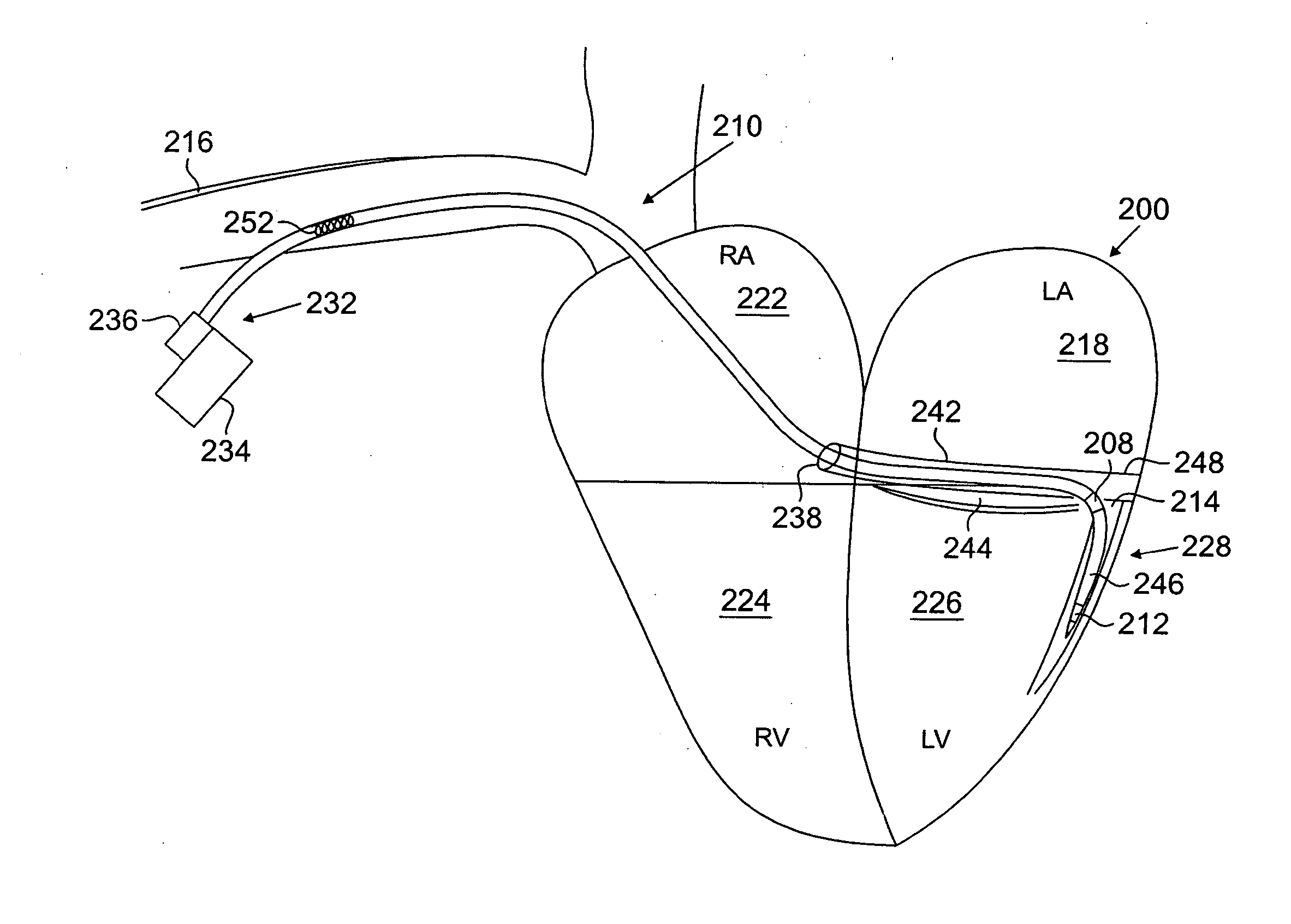
[0039] In still another embodiment, an LV motion
mapping system is disclosed which can sense LV motion for optimizing CRT
lead placement. The system may include an LV venous catheter, LV lead, guidewire, or
guide catheter / catheter system with an acceleration sensor, connected to a
signal processing and powering module, and a
graphical display. The acceleration sensing catheter may be moved to different locations in the LV and used to identify regions of late systolic or post-systolic motion relative to a reference point such as the QRS, valve closures, or isovolumic contraction / relaxation. Alternatively, a pacing catheter or guidewire may be moved to different LV locations and an acceleration sensing catheter near the
mitral annulus may measure changes in LV function due to pacing. Both techniques may be used to optimize CRT LV lead implantation. The
mapping system may also be used to determine optimal RV pacing sites which may mitigate the need for placing an LV CRT lead.
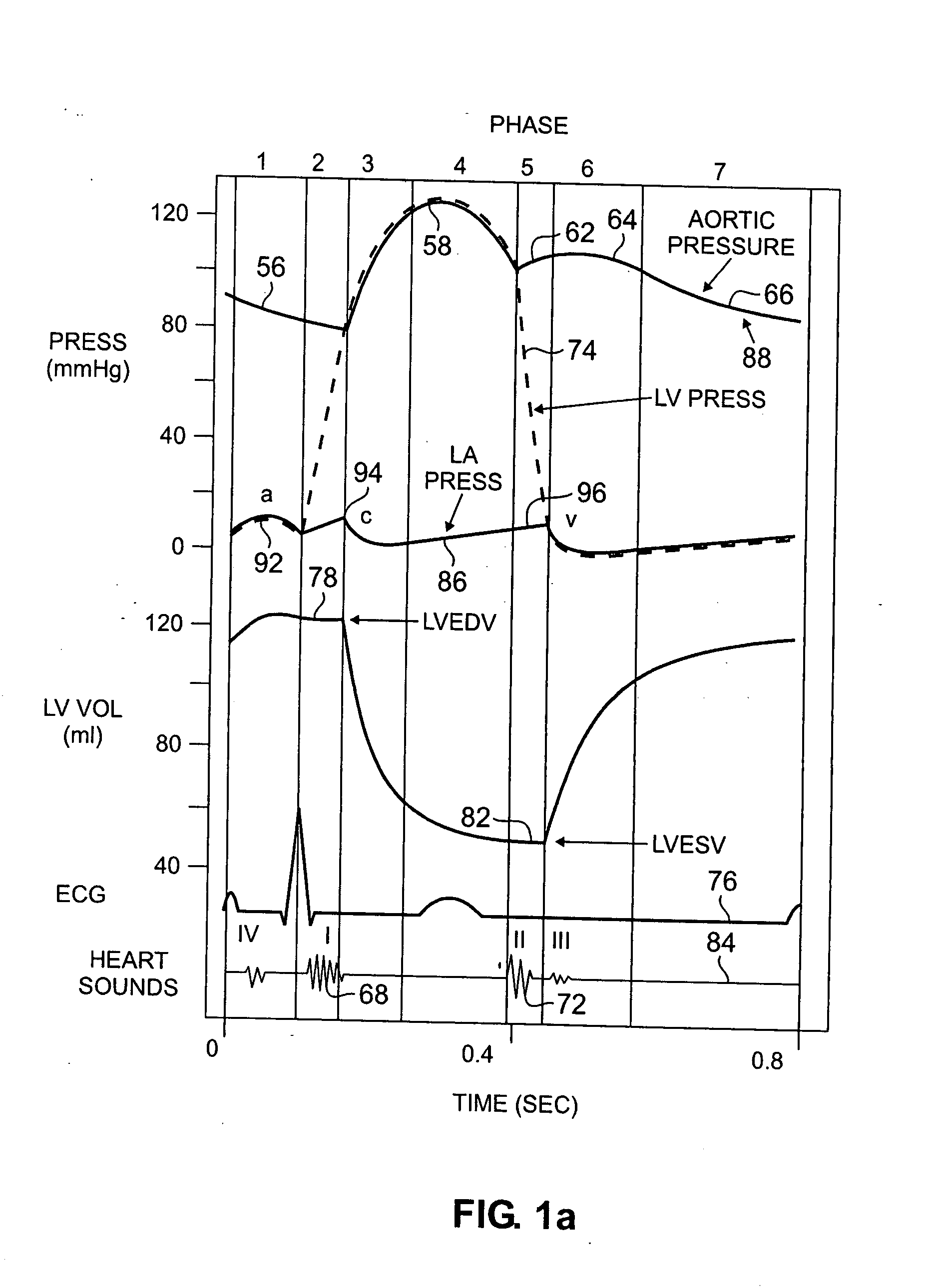
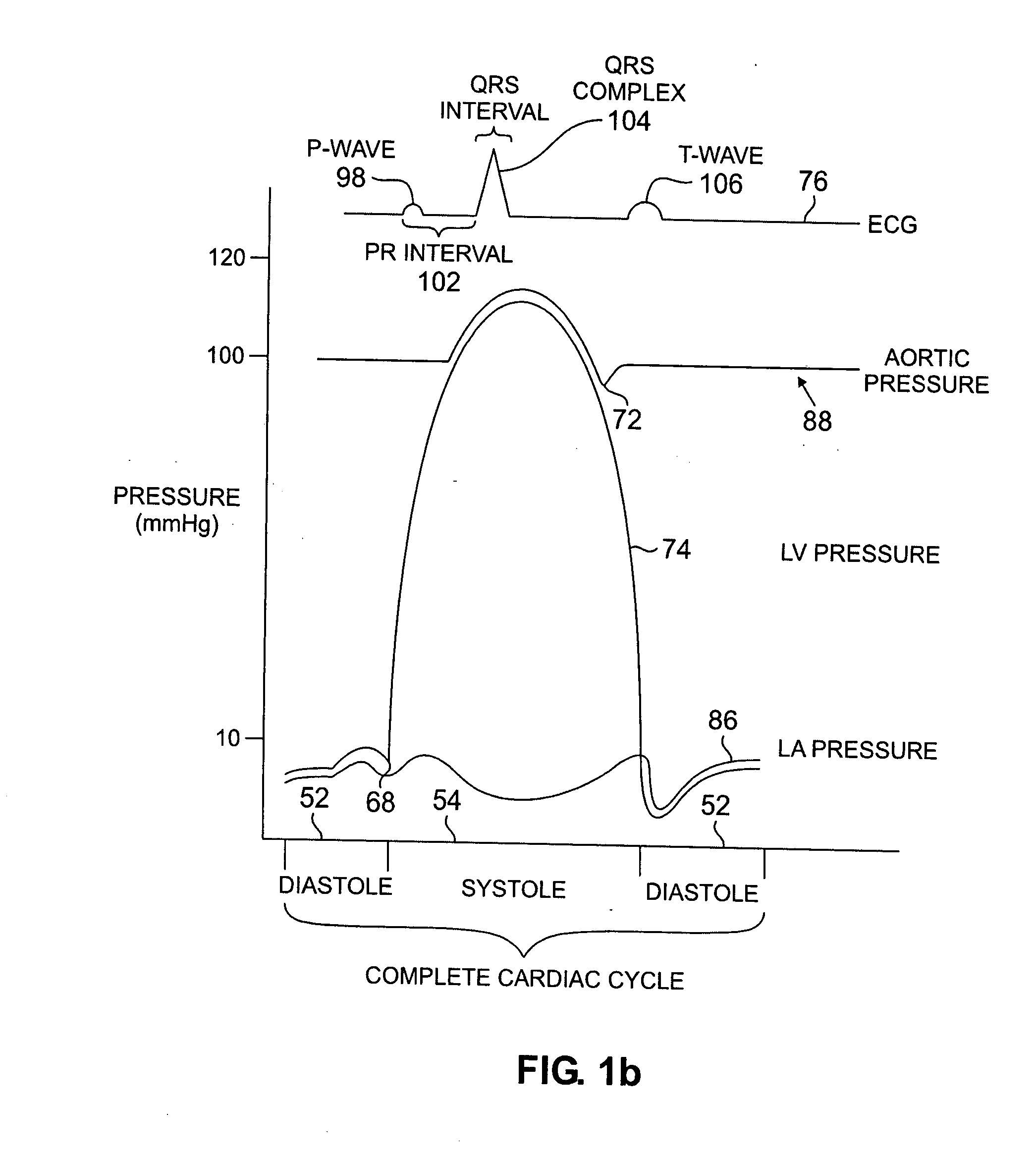
[0041] Monitoring changes in the frequency of the vibrational component of the ICP may be more practical and accurate than measuring amplitude changes for assessing cardiac function. Similarly, monitoring the time interval of this phase may prove more practical and accurate. Such an approach would reduce the sensitivity of the acceleration
signal measurement and the interpretation of this measurement to the effects of gravity, sensor axis orientation,
relative motion of the sensing device to which the sensor is affixed, and
translational motion of the heart.
 Login to View More
Login to View More  Login to View More
Login to View More