Method for synthesis of titanium dioxide nanotubes using ionic liquids
a titanium dioxide nanotube and ionic liquid technology, applied in the field of titanium dioxide anodization methods, can solve the problems of limited electrical conductivity of electrolyte solutions used in anodization processes of the art, difficulty in anodization process of the art, etc., and achieves improved pv properties, less energy consumption, and high growth rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of TiO2 Nanotubes
[0098]The ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate (BMIM-BF4) was mixed with deionized water in a BMIM-BF4: water weight ratio of 0.276: 1, and the resulting fluid used as the electrolyte. The anodization process was conducted on a 0.5 mm thick pure titanium foil (purchased from McMaster-Carr) using a two-electrode DC power supply with a voltage output range of 0-40 V. The titanium foil surface was used as-received (milled) and cleaned with acetone followed by an ethanol rinse before the synthesis. The titanium foil was connected as the working electrode and a piece of platinum mesh was used as the counter electrode. The synthesis was at room temperature, i.e., about 21° C. A constant potential of 10 volts was applied during the synthesis. The time-dependent anodization current was monitored using a multimeter. The current was about 2 mA at the beginning of the synthesis and gradually decreased and stabilized at a level of 0.5 mA for 80...
example 2
Microscopic Analysis of the TiO2 Nanotubes
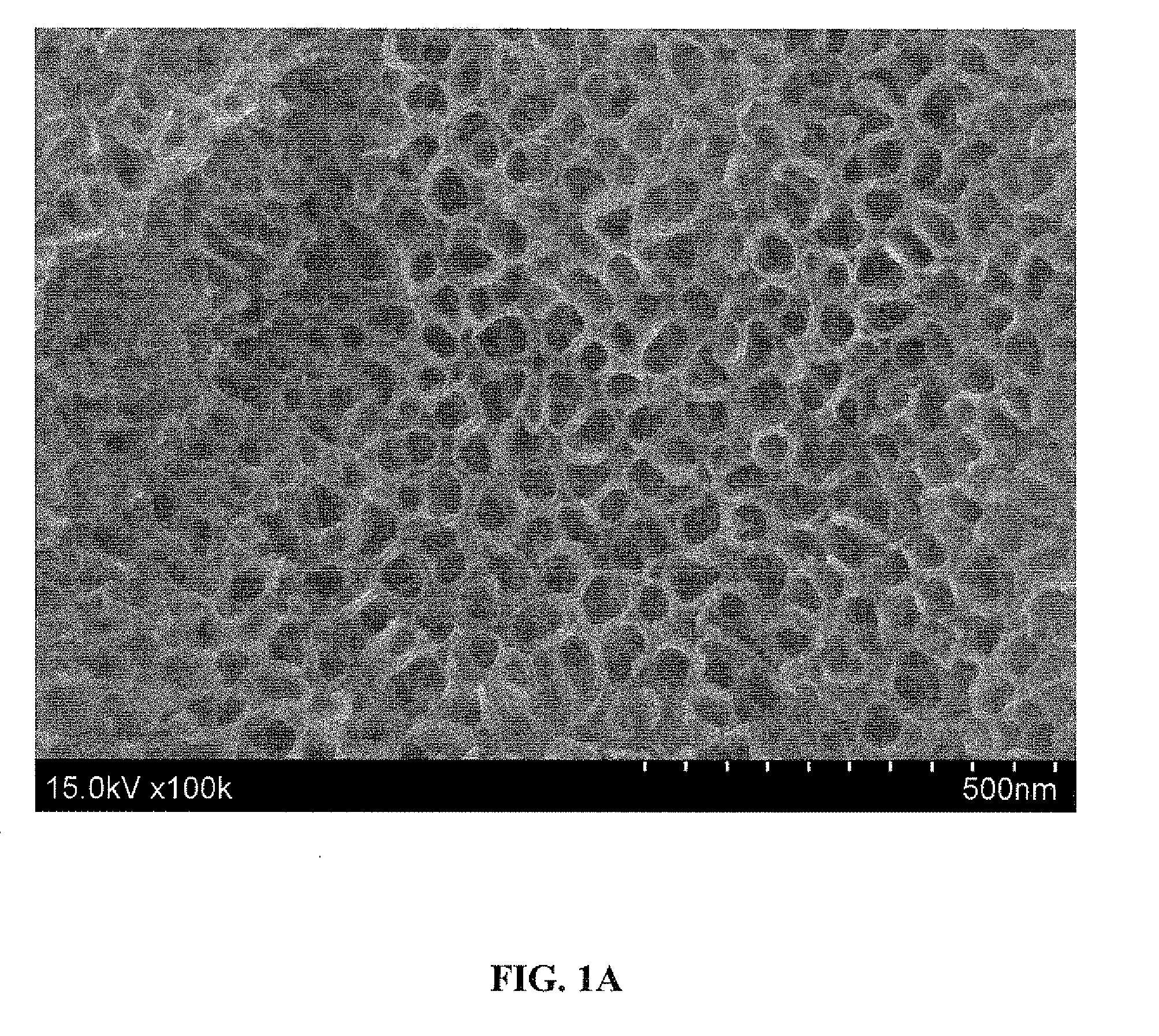
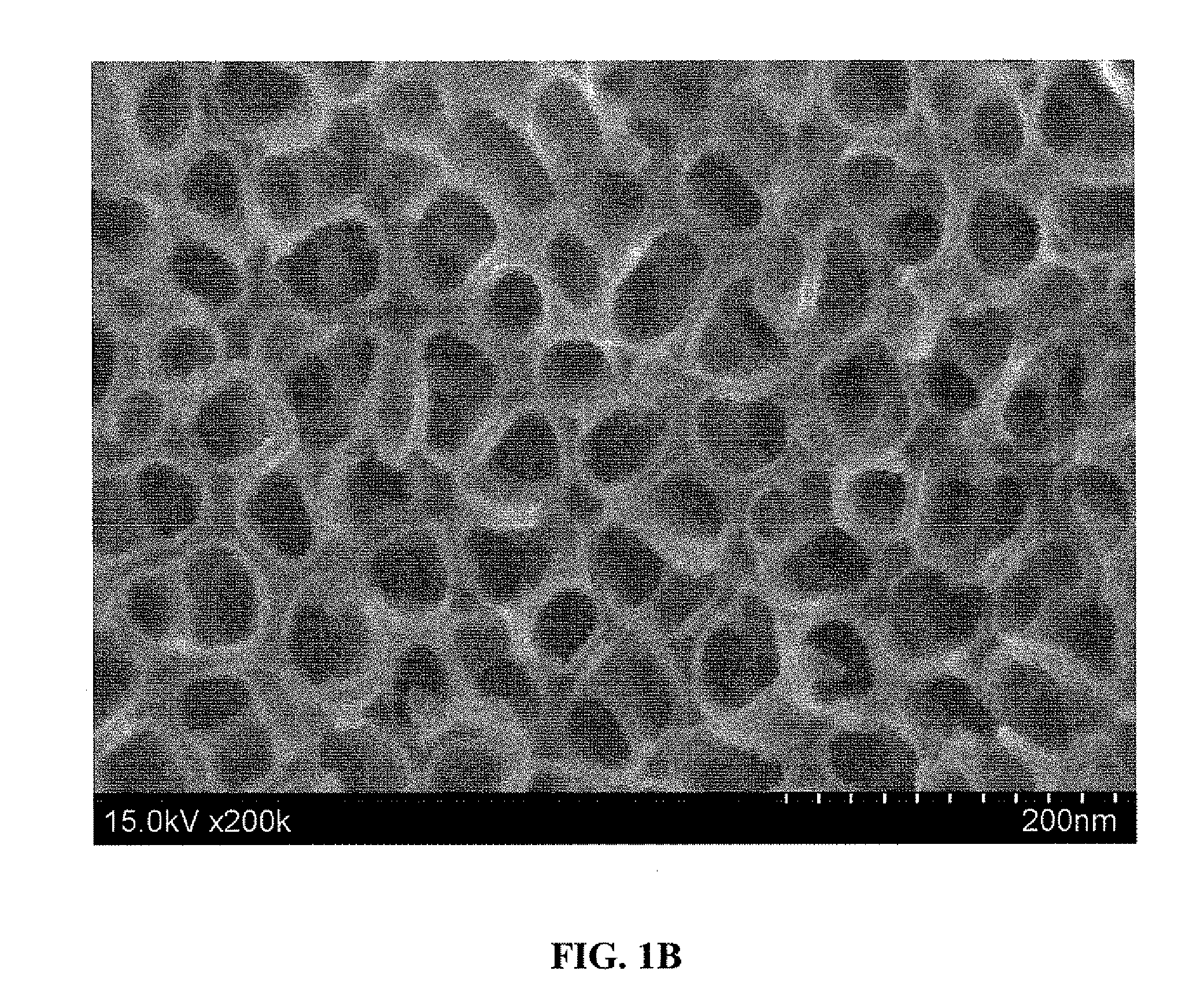
[0099]The as-anodized titanium surface prepared according to Example 1 above was analyzed by scanning electron microscopy (SEM). Before imaging, the surface was ultrasonically cleaned in acetone for 10 minutes during which time the top oxide layer was spalled off to expose the nanotubes underneath.
[0100]FIGS. 1A and 1B show 100,000× and 200,000× magnified, respectively, top view SEM micrographs of the surface. As shown, the SEM images show a thin, dense layer containing a highly ordered array of TiO2 nanotubes covering the as-anodized titanium surface. The nanotubes are shown to have a pore size of 25-35 nm and an outer tube diameter of 40-55 nm.
[0101]FIGS. 2A and 2B show 50,000× and 100,000× magnified, respectively, cross-sectional SEM micrographs of the surface. As shown, the nanotubes have a length of 250-350 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com