Mutant blue fluorescent protein and method of using the same for fluorescence resonance energy transfer and blue fluorescent fish
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
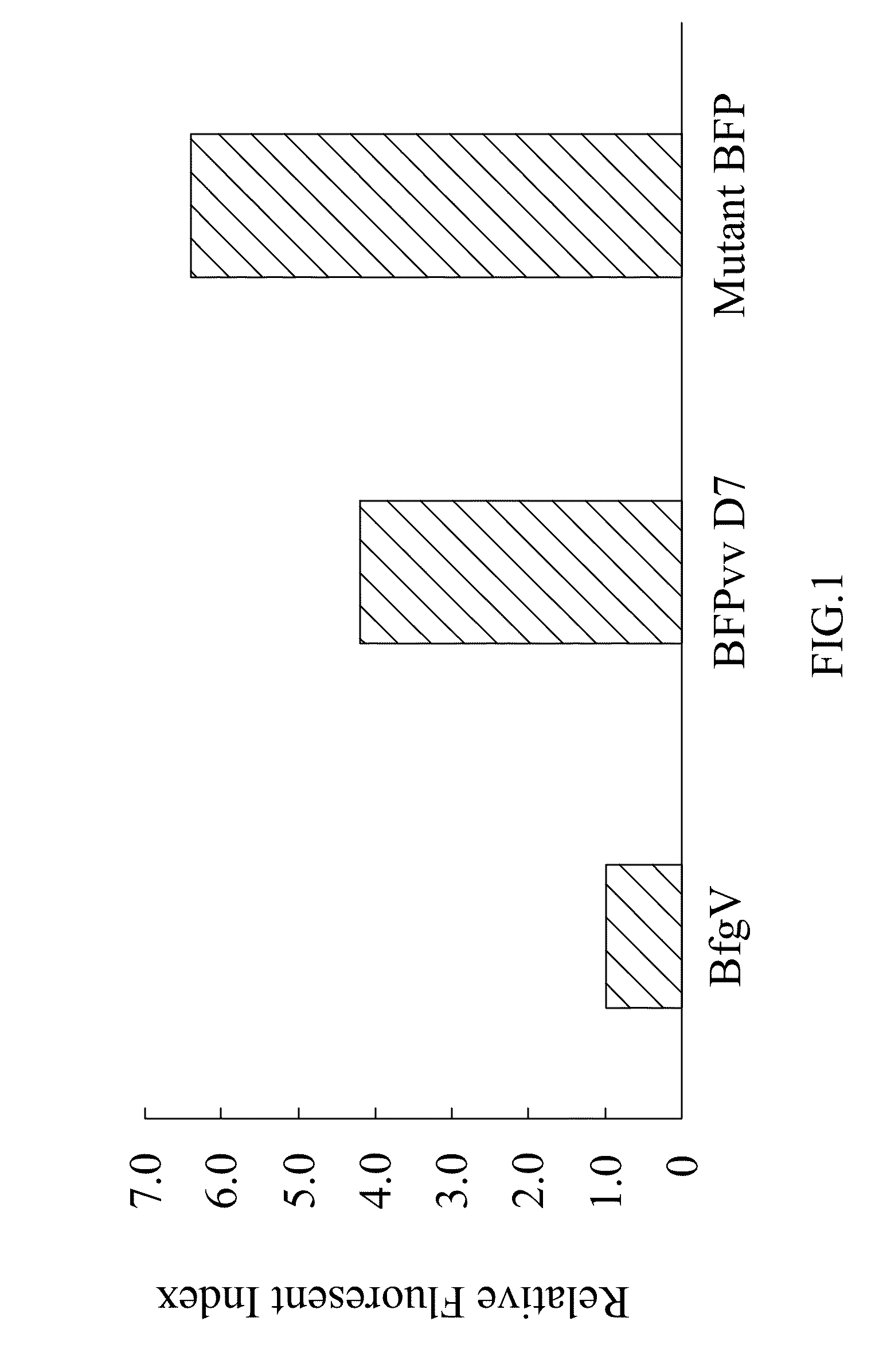
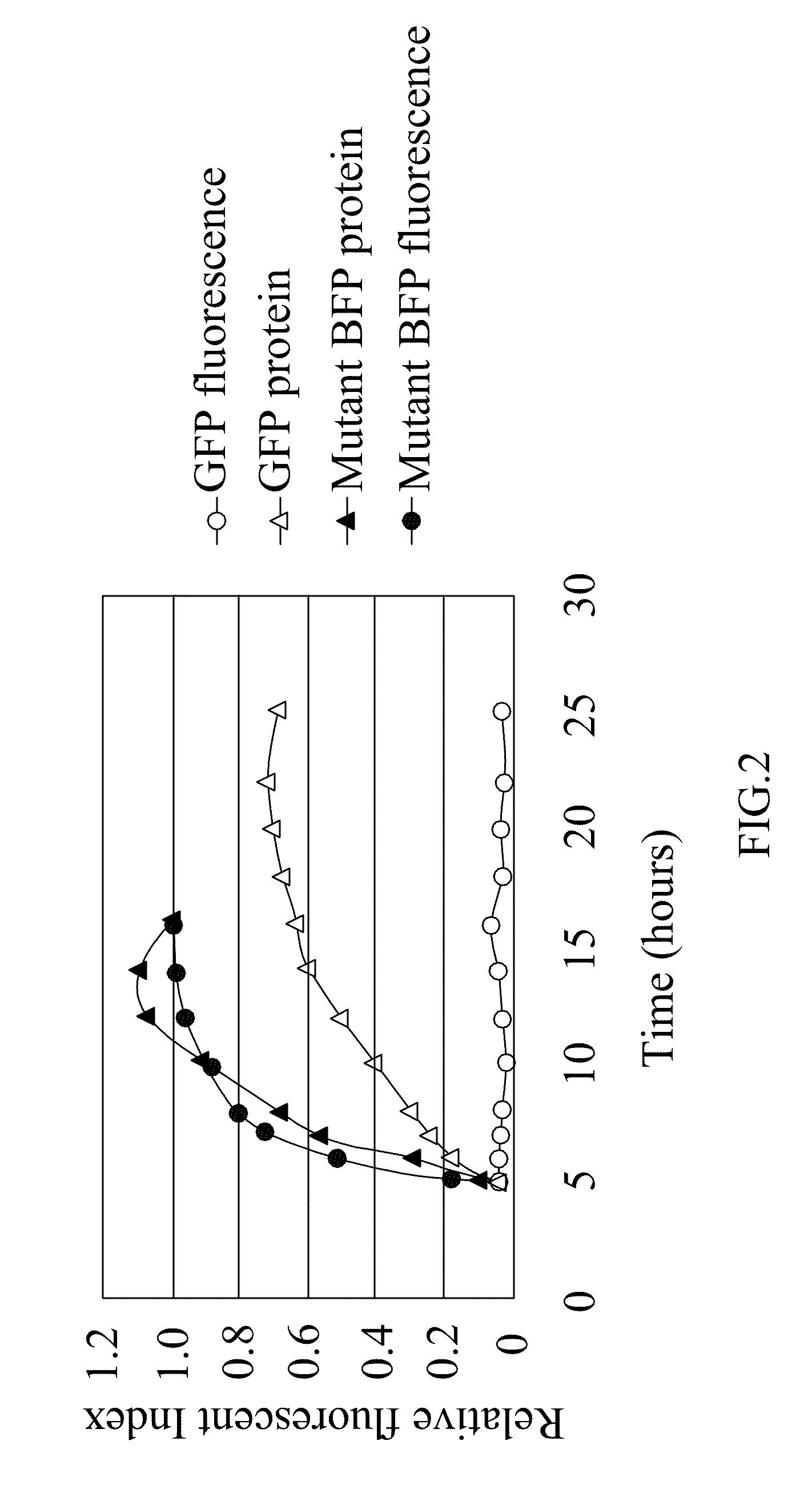
[0043]The term “mutant blue fluorescent protein (BFP)” as used herein refers to the upgrade from the BFPvv D7 of SEQ ID NO:2 derived from the wild type BFP, BfgV of SEQ ID NO:1, obtained from Vibrio vulnificus.
Construction of Mutant BFP
[0044]The present invention provides a mutant blue fluorescent protein (BFP) that can be mutated by an error-prone PCR method or a DNA shuffling method with using the BFPvv D7 of SEQ ID NO:2 as parents as described as follows:
[0045]Bacterial Strains and Growth Medium: E. coli BL21(DE3) (Stratagene; CA, USA) was used as a host for gene expression and screening work. Bacteria used in the present invention were raised in Luria-Bertani (LB) broth or on LB agar. When required, 100 μg / ml ampicillin was added into the medium. Isopropylthio-b-D-galactoside (IPTG) was used as inducer at 1 mM in broth and 0.1 mM in agar plates. All medium components were purchased from Difco (MI, USA) and chemicals were from Sigma (MO, USA).
[0046]Plasmid Construction: A 751-bp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com