Surface modification of polymers via surface active and reactive end groups
a technology of reactive end group and surface modification, which is applied in the direction of synthetic polymeric active ingredients, drug compositions, extracellular fluid disorder, etc., can solve the problems of difficult removal of organic solvent after removal, uncontrolled degradation process, and significant ablation tendency of base polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
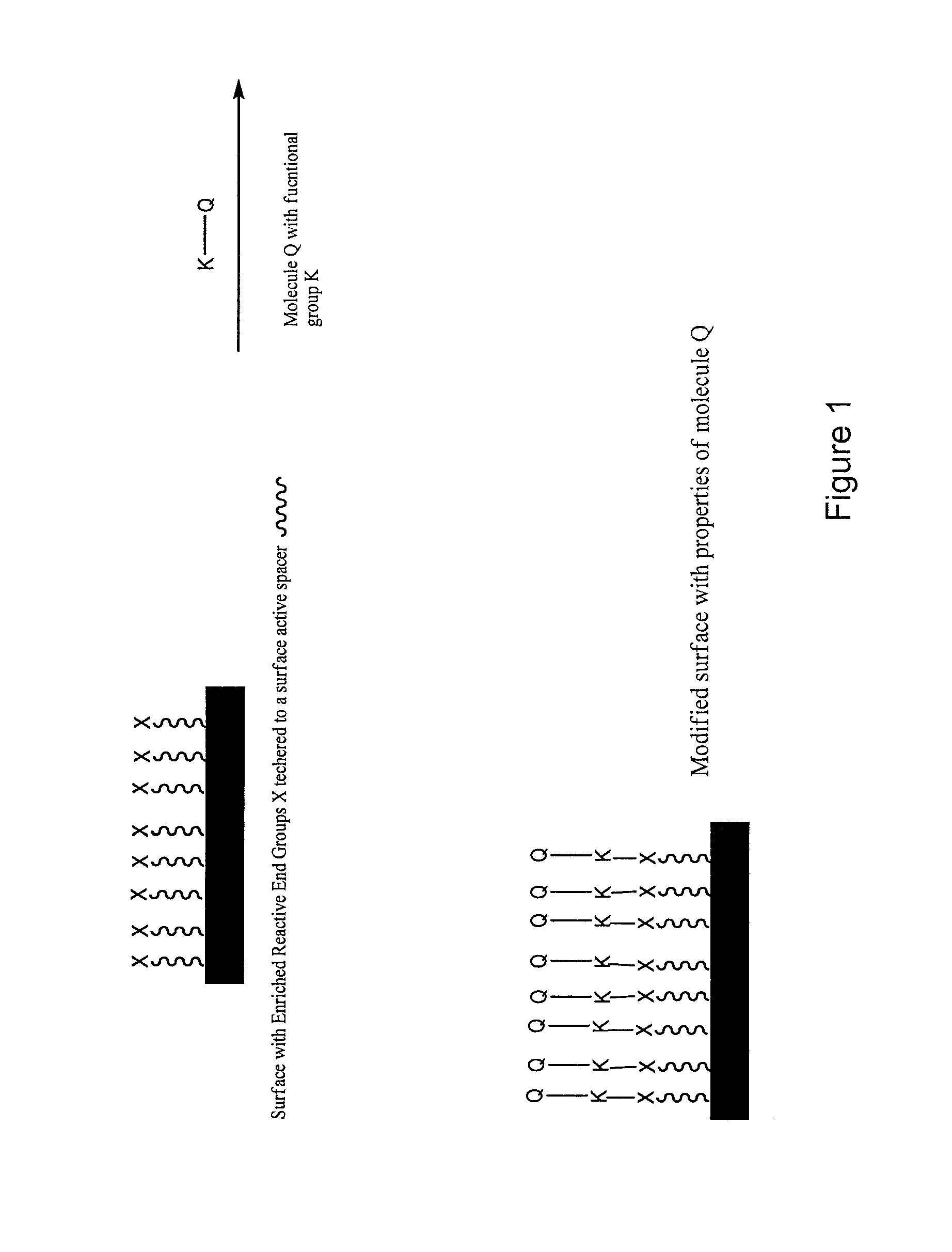
[0067]Using surface active and reactive diamine as an end capping agent, a polyurethane with amine terminated end groups can be prepared by a two-step method: I) First, isocyanate terminated polyurethane was prepared in DMAc solution from diisocyanate such as MDI, polyol such as PTMO, PEO, and polyol such as polycarbonate diol, silicone diol, and chain extender such as butane diol, ethylene diamine, ethanol amine, and other short chain diamine, diol, and amino alcohol. The stoichiometric ratio of NCO / H was kept more than 1 so that the polyurethane chain ends were terminated with isocyanate groups, II) Excess amount of surface active and reactive diamine was then added to the reaction mixture to allow the covalent attachment of these end groups at one site, leaving the other amine group for subsequent surface modification. The polymer thus prepared can be used as a coating or can be precipitated and dried for thermal processing such as extrusion, molding. Because of the surface activ...
example 2
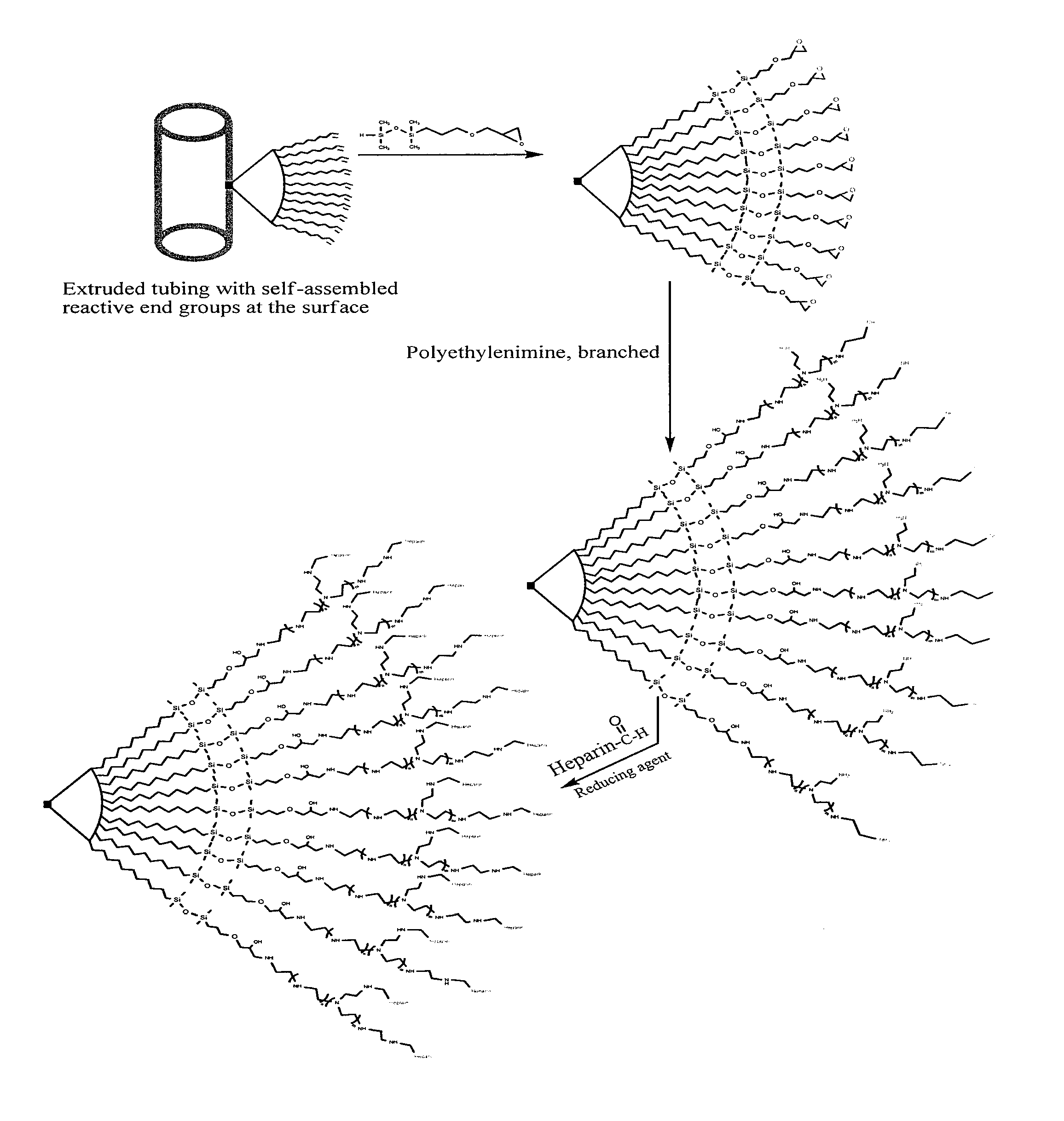
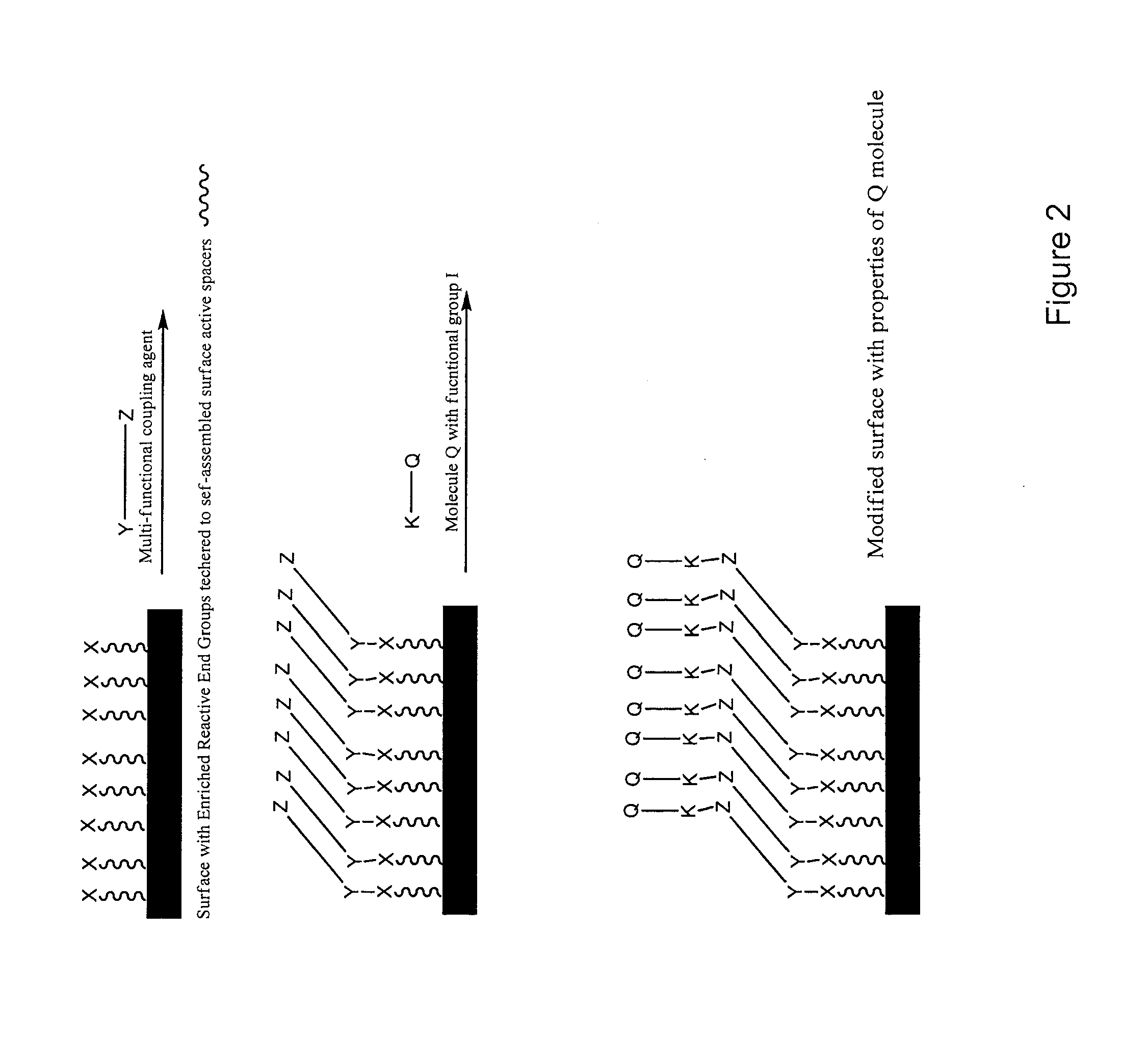
[0070]Polyurethane with 10-undecen-1-ol end groups was first prepared. Tubing was extruded from the resin having the surface enriched with the vinyl end groups which were then reacted with an epoxy silane coupling agent to form a surface abundant with epoxide, the epoxide functional surface can serve as platform for immobilization of hydrophilic molecules such as PVP, PEO, PVA, PMA, polyelectrolytes, and other biomolecules bearing functional group reactive to epoxide to afford wet lubricity. Applying multifunctional hydrophilic molecules may also lock-in the surface with desired properties. Reaction may also take place with underlying reactive end groups due to the penetration / diffusion of surface modifying agents, these underlying end groups will serve as the reservoir for replenishing the surface in demand. Alternatively, the epoxy groups can react with polyamine to form an amine rich surface which can serve as platform for immobilization of biomolecules such as commercially avail...
example 3
[0073]Hydroxyl-functional surface active and reactive end groups capping agents can also be optimized and incorporated in polyurethanes as surface active and reactive end group. Examples of such compounds are 11-(9-decenyldimethylsilyl)undecan-1-ol and 11-(triallylsilyl)undecan-1-ol and 11-(triallylsilyl)undecan-1-ol. The synthesis of the molecules is described below:
Synthesis of 11-(9-decenyldimethylsilyl)undecan-1-ol
[0074]The title compound was synthesized as illustrated in FIG. 5. 10-undecen-1-ol (85 g, 0.5 mol) and p-toluenesulfonic acid monohydrate (0.38 g, 2 mmol) were dissolved in dichloromethane (150 mL) and cooled in ice / water bath under nitrogen. To this solution was then added 3,4-dihydro-2H-pyran (50.4 g, 0.6 mol) dropwise over an hour. After the addition, the solution was stirred for additional two hours in ice / water bath and turned into purple. The solution was then diluted with hexanes (300 mL), washed with aqueous sodium bicarbonate (150 mL×2), and dried over MgSO4. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| contact angle | aaaaa | aaaaa |
| water contact angle | aaaaa | aaaaa |
| tensile strengths | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com