High-yield activation of polymer surfaces for covalent attachment of molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of RGD-Modified Nylon Substrate
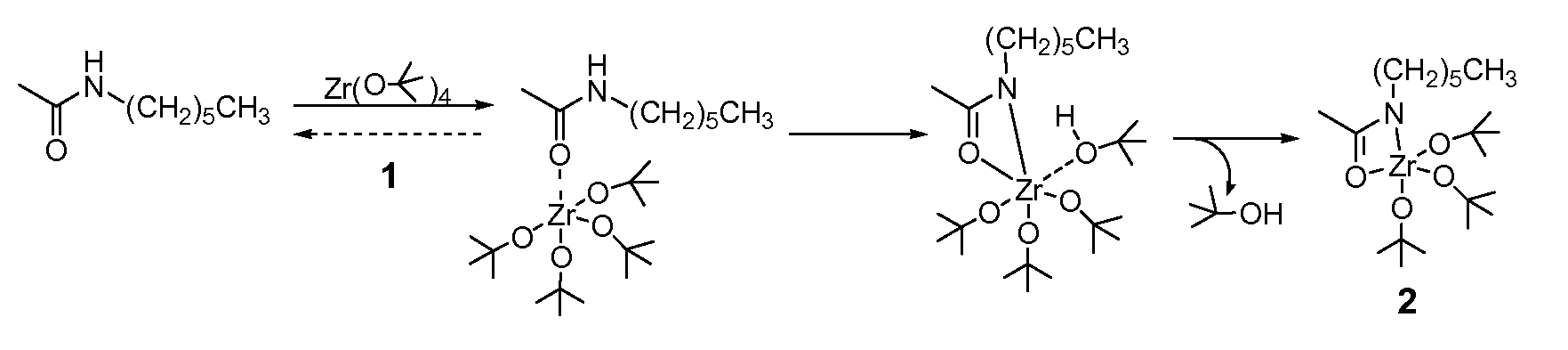
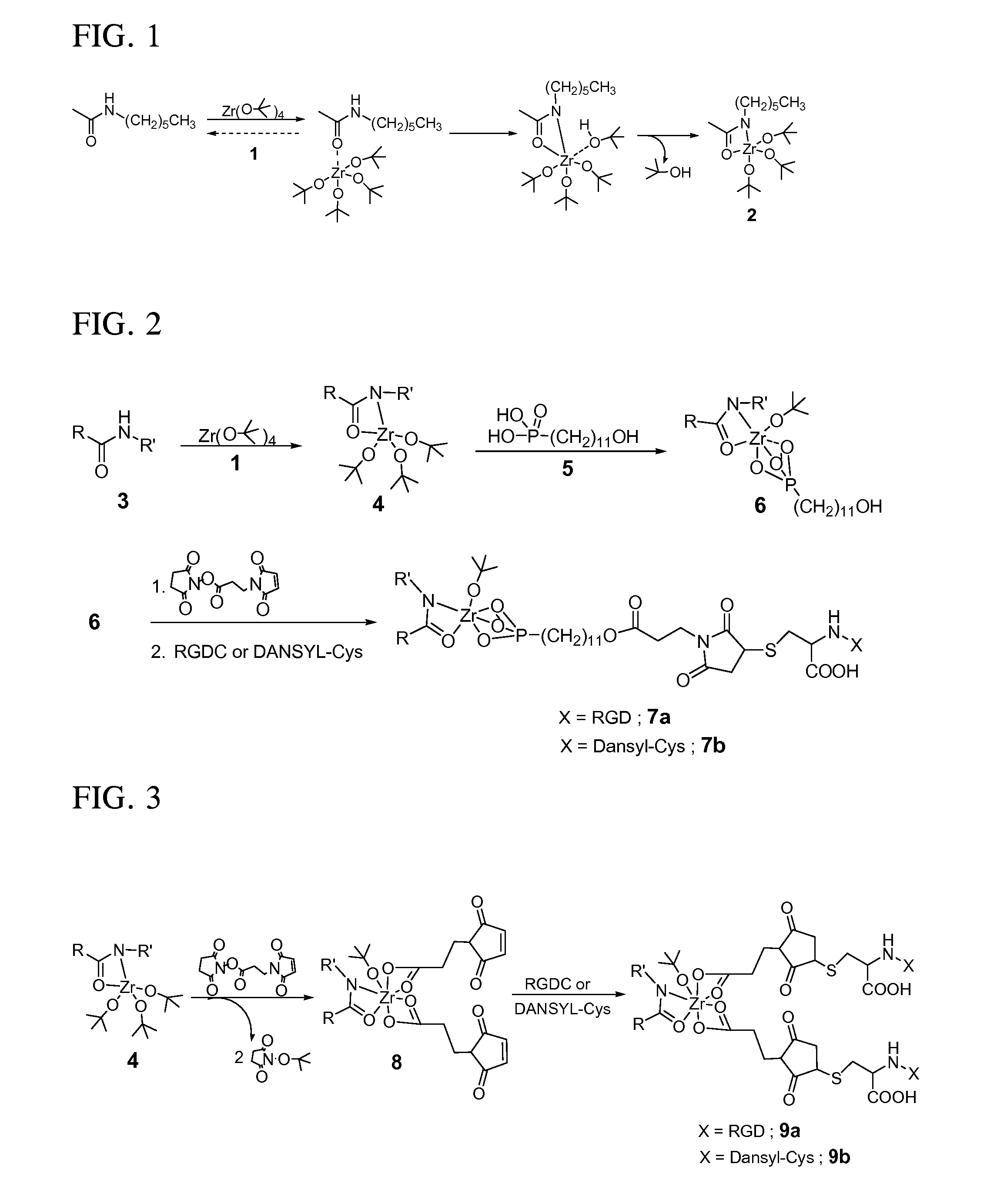
[0129] Activated nylon surface 4 was prepared by first casting films of 3 (nylon 6 / 6; (R═(CH2)4CO; R′═(CH2)6NH) from 0.1 mM formic acid solution on glass microscope slides that were rinsed copiously in Millipore® water, and evacuated at 10−2 torr for 3 hours. The coated slides were then placed in a deposition chamber that was equipped with two stopcocks for exposure either to vacuum or to vapor of zirconium tetra(tert-butoxide). The chamber was evacuated to 10−3 torr for 30 minutes, and slides of 3 were exposed to vapor of of zirconium tetra(tert-butoxide) (with external evacuation) for 30 seconds followed by 5 min exposure without external evacuation. This cycle was repeated twice, then followed by an additional 10 minutes of exposure without external evacuation. The chamber was then evacuated for 16 hours at 10−3 torr to ensure removal of excess zirconium tetra(tert-butoxide). The IR spectrum of polymer surface-bound Zr complex (4) showe...
example 2
Preparation of RGD-Modified Nylon Substrate
[0131] RGD-derivatized surface 9a (FIG. 3) was prepared by immersing a 4-coated slide in a 0.1 mM solution of 3-maleimidopropionic acid N-hydroxysuccinimide ester in dry acetonitrile for 16 hours to produce 8. Immersion of 8 in a 0.1 mM aqueous solution of RGDC at pH 6.5 for 24 hours produced 9a. The nylon-Zr surface complex was derivatized with the succinimide ester of 3-maleimidopropionic acid directly by transesterification to produce 8, which can result in either an RGDC or DANSYL-Cys-tethered surface (9a or 9b). Complexes 9a and 9b have a 1:2 ratio of zirconium to RGDC or DANSYL-Cys, respectively.
example 3
Preparation of DANSYL-Cys-Modified Nylon Substrate
[0132] Fluorescent molecule-labeled analogues 7b and 9b were prepared as described for 7a and 9a, but a 0.1 mM aqueous solution of N-(5-(dimethylamino)-1-naphthyl-sulfonyl)-cysteine (DANSYL-Cys) was used instead of RGDC (FIGS. 2 and 3).
[0133] To address the issue of solvent-induced polymer swelling, control films of 3 were prepared by soaking in 0.1 mM DANSYL-cys solution for 24 hrs. A calibration curve of fluorescence intensity versus concentration was measured for DANSYL-Cys solutions from 0.16 to 21 μM at pH 7.5 and pH 12.
[0134] Surface complex DANSYL content of 7b and 9b was quantified by immersion in water at pH 12 for 3 hours, which cleaves the Zr complexes from the surface, precipitates ZrO2, and releases fluorophore from 7b and 9b into solution. The amount of DANSYL surface-bound through Zr complexes 7b and 9b was measured to be 0.10 nmol / cm2 and 0.18 nmol / cm2, respectively. These amounts are consistent with the DANSYL:Zr ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Structure | aaaaa | aaaaa |
| Inductive effect | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com