Process for the production of chemicals
a chemical and process technology, applied in the direction of sustainable manufacturing/processing, indirect fuel cells, final product manufacturing, etc., can solve the problems of large electrical energy consumption for aeration, high cost, and high cost of chemical catalysts, so as to avoid unsatisfactory increase in ph in the cathode compartment and maintain homeostasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Biopolymer Production
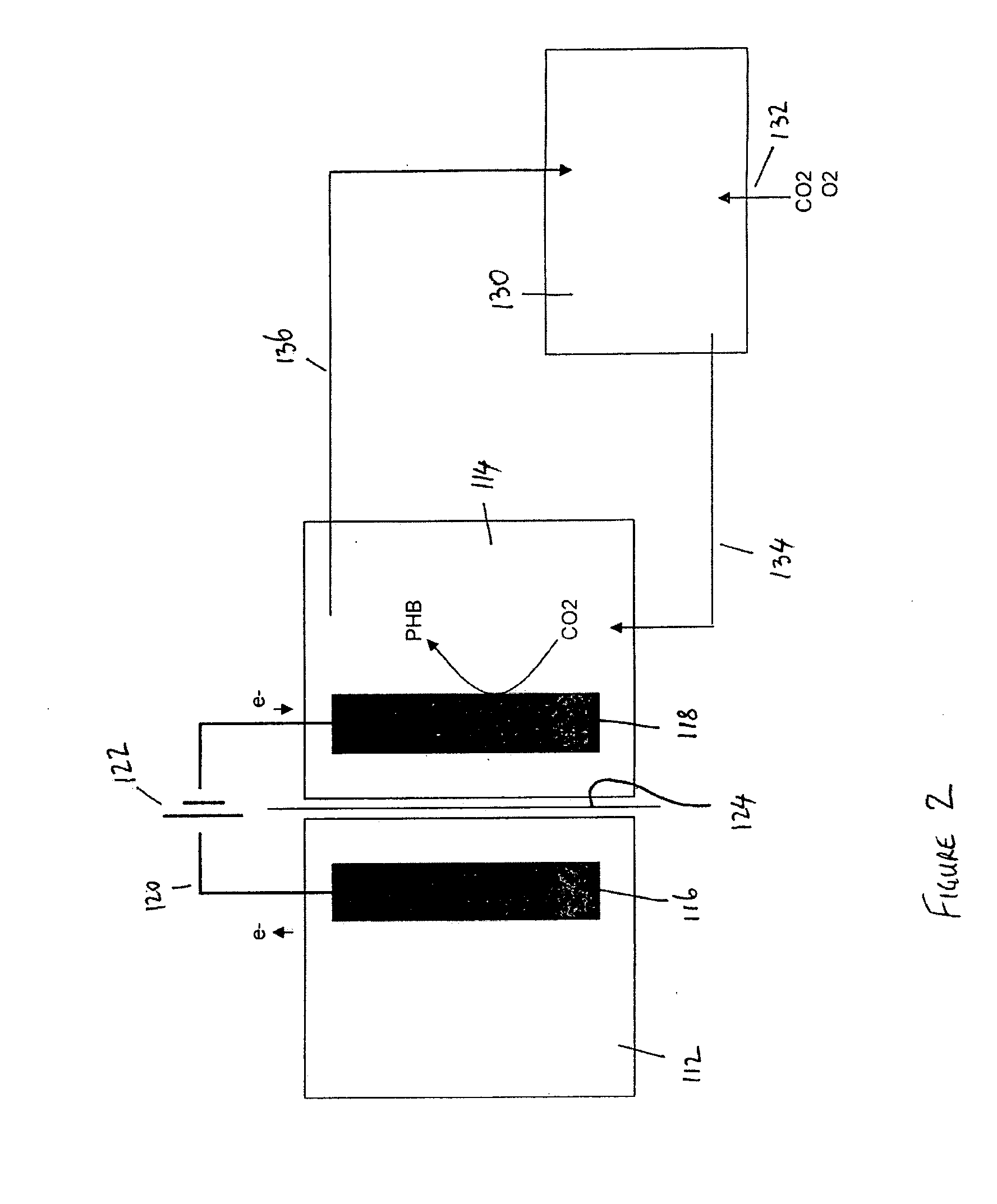
[0072]In this example, which uses the apparatus as shown in FIG. 2, bacteria in the cathode chamber use carbon dioxide and electrons from the cathode as energy and carbon source, in which case they can produce biopolymer under the form of poly-β-hydroxybutyrate (PHB). The CO2 is provided in a way that a pure culture or a defined mixture of bacteria can be maintained. Oxygen is supplied to support the PHB synthesis. The electrons reach the bacteria either directly or indirectly through e.g. the production of hydrogen at the cathode. An external power source can provide the required additional reducing power at the cathode, if required
[0073]Example organism in the cathode: Cupriavidus necator (formerly Alcaligenes eutrophus or Ralstonia eutropha)
example 2
Indirect Provision of Reducing Power to Biochemicals Producing Organisms
[0074]In this example the apparatus as shown in FIG. 3 is used and a redox shuttle is reduced in the cathode compartment. The reduced redox shuttle is brought to the external compartment (possibly through a permeable membrane) where micro-organisms use the reduced redox shuttle as electron donor for the reduction of an electron acceptor, being CO2, and the production chemicals from this CO2. An external power source can provide the required additional reducing power at the cathode, if required
example 3
Reuse of CO2 Produced at the Anode to Drive the Cathodic Reaction
[0075]This example is conducted in the apparatus as shown in FIG. 4. The anode contains micro-organisms that oxidize a carbon source. The CO2 produced is stripped in situ, or in an external stripping reactor, and hence brought to the cathode compartment in such way that the cathode compartment can contain a well defined culture or mixed culture of micro-organisms to form the desired chemicals.
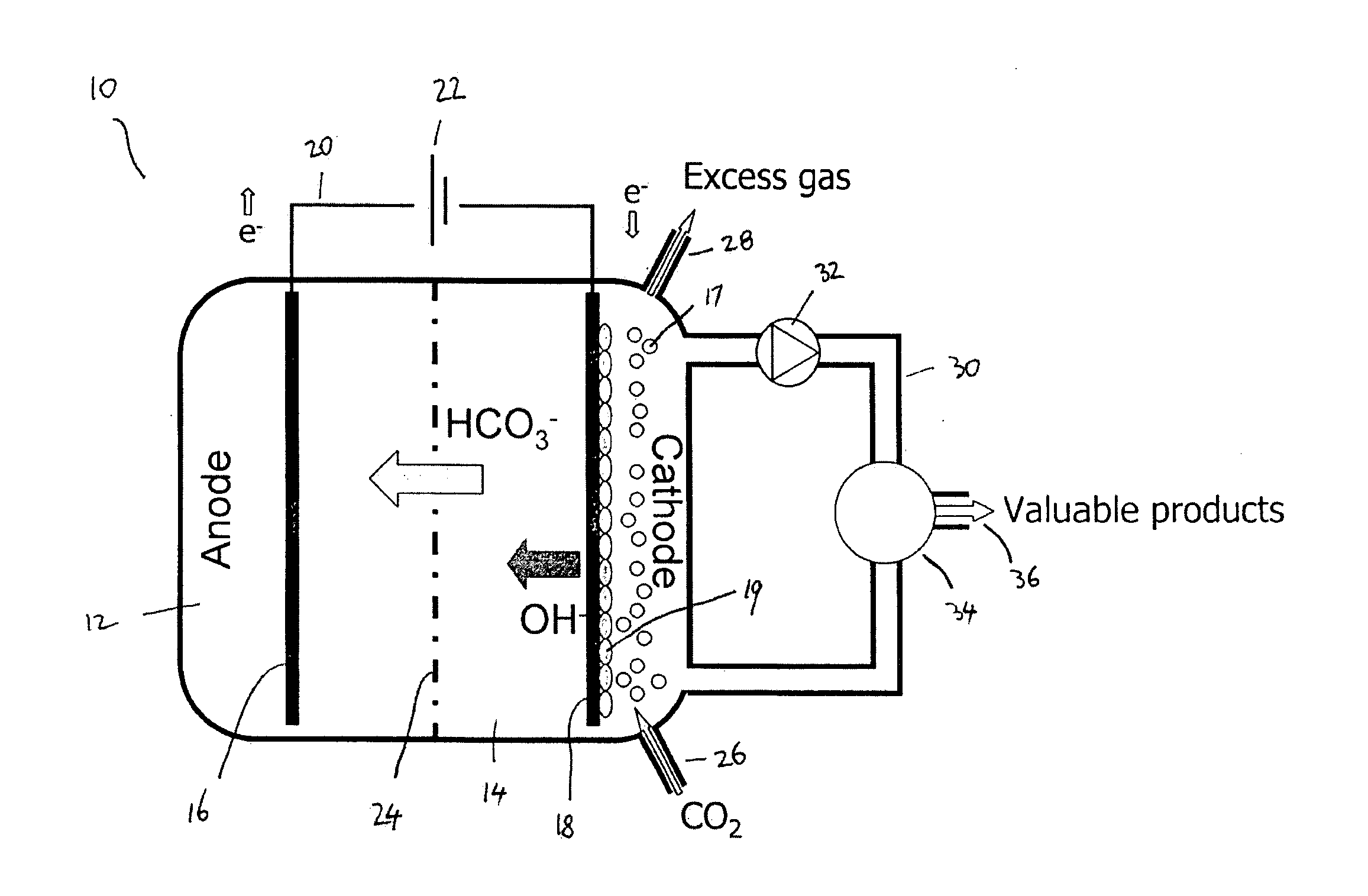
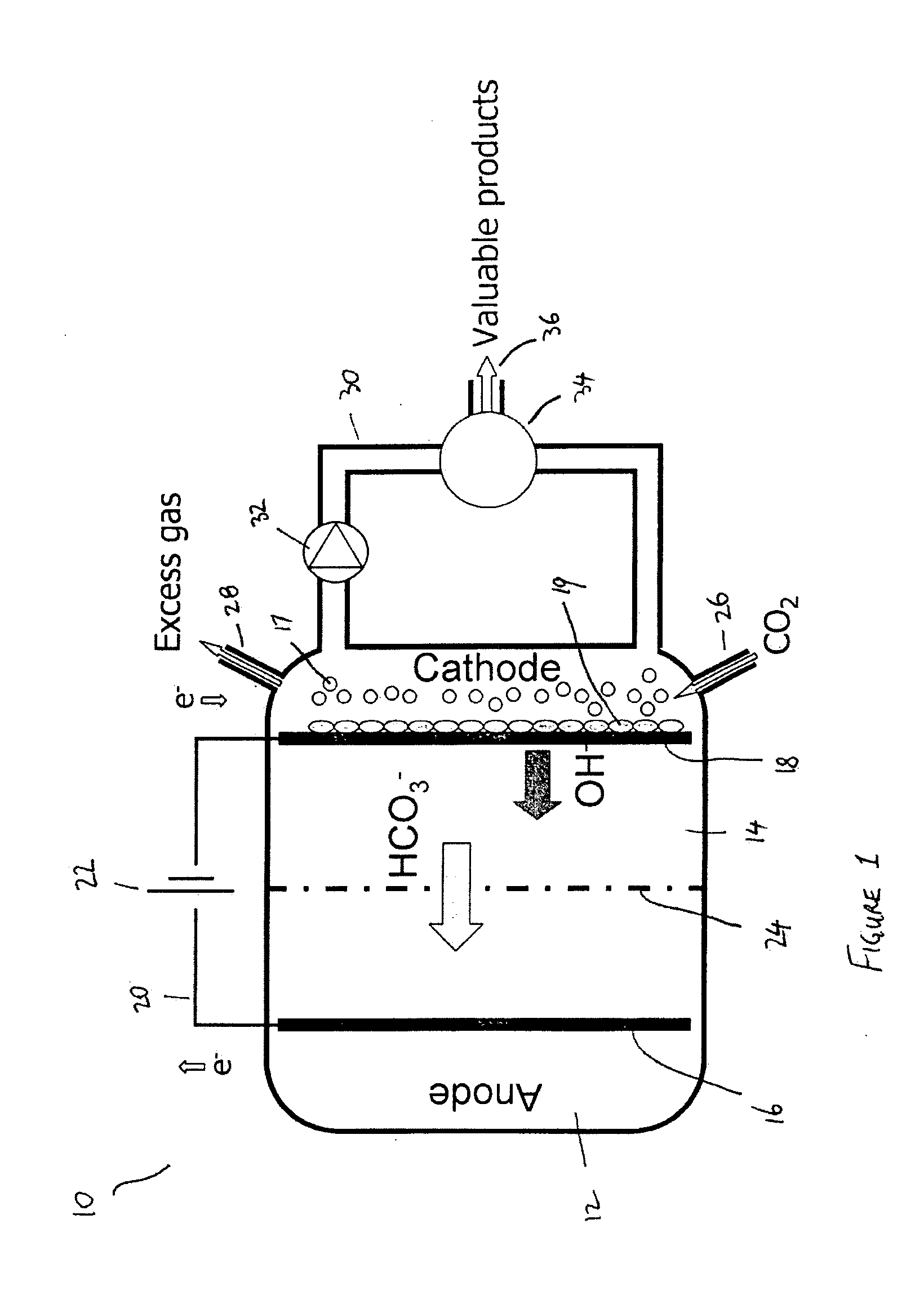
[0076]The present invention presents a cathode system for producing complex molecules using microbial biocathodes prevents the abovementioned problems associated with the contamination of unwanted micro-organisms and / or cathode chamber pH increase and / or salinity increase.
PUM
| Property | Measurement | Unit |
|---|---|---|
| area specific current density | aaaaa | aaaaa |
| area specific current density | aaaaa | aaaaa |
| area specific current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com