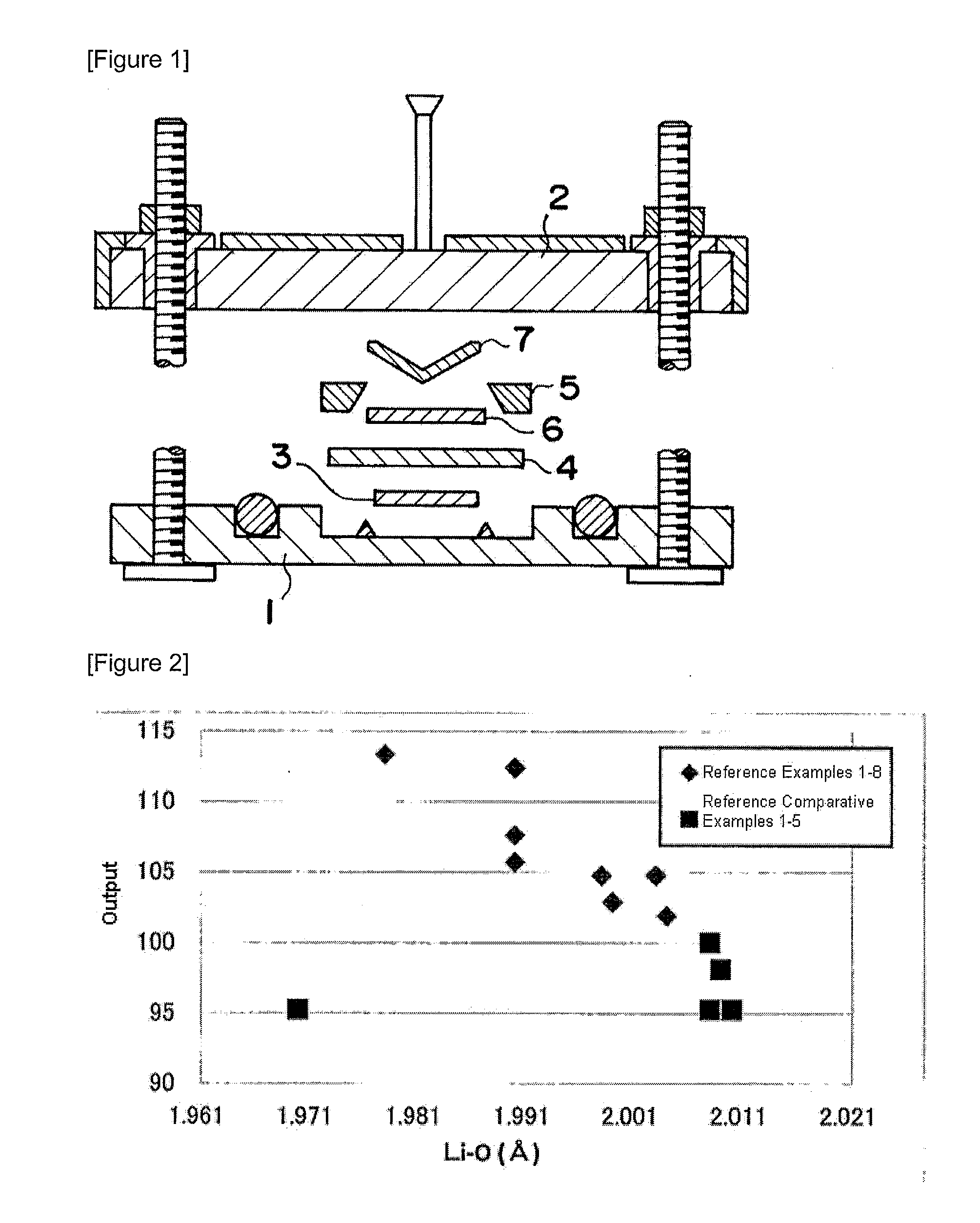
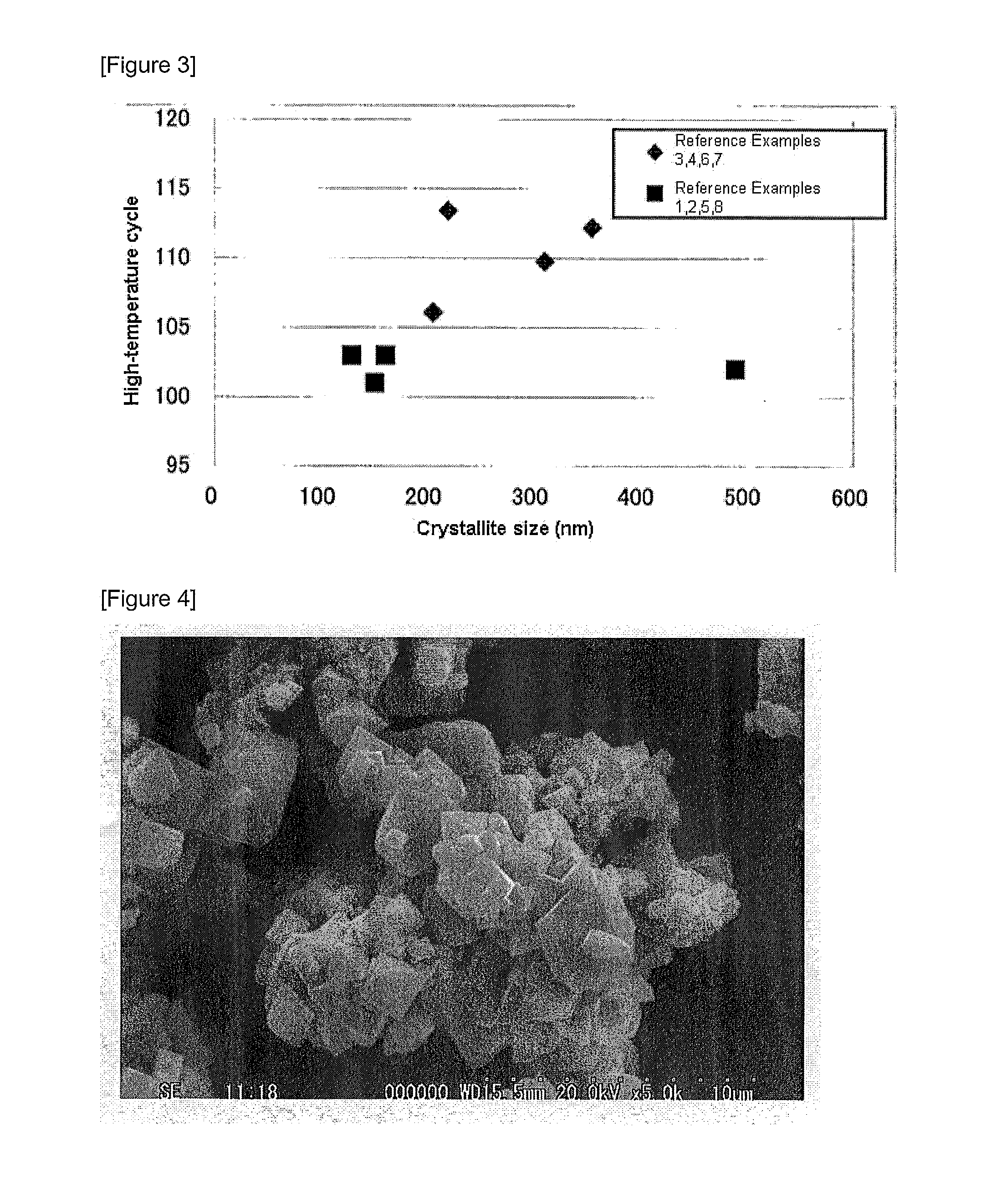
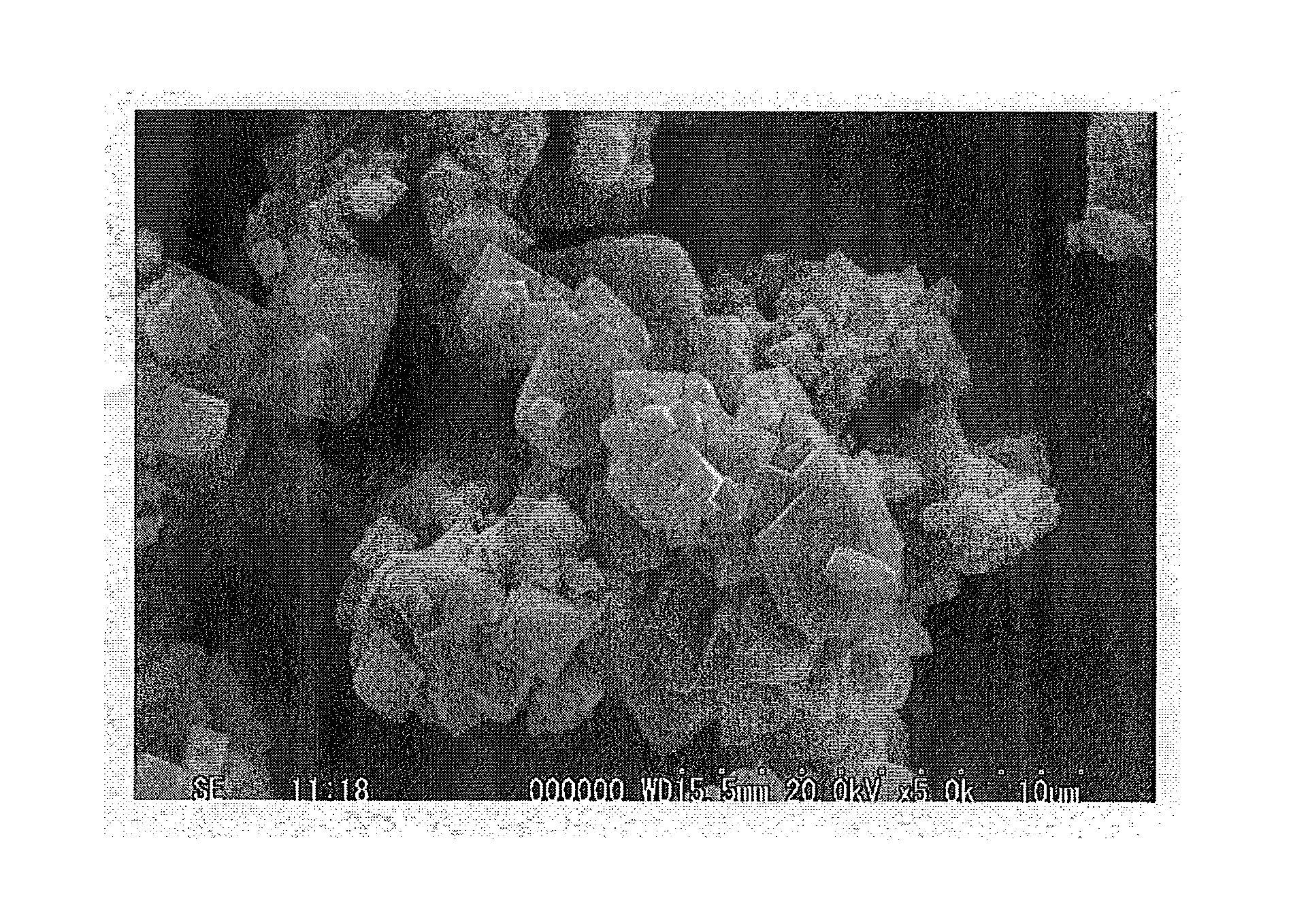Positive Electrode Active Material for Lithium Battery
a lithium battery and active material technology, applied in the direction of magnetic paints, cell components, magnetic bodies, etc., can solve the problems of difficult to obtain output, achieve the effect of preventing short circuits (voltage drops), increasing output characteristics, and difficult to obtain output characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0116]In the following, the present invention will be described further based on examples; however, the present invention is not to be limited to the examples indicated below.
[0117]For the samples (powders), the inter-atomic distances Li—O and Mn—O and the crystallite size were measured by the Rietveld method using the fundamental method described in the following.
[0118]The Rietveld method using the fundamental method is a method whereby the structural parameters of a crystal are refined from the diffraction intensities obtained by powder x-ray diffraction or the like. It is a method in which a crystal structure model is hypothesized, and various parameters of this crystal structure are refined in such a way that the x-ray diffraction pattern derived by calculations from this structure matches as much as possible the actually measured x-ray diffraction pattern.
[0119]An x-ray diffractometer (D8 ADVANCE, manufactured by Bruker AXS) using a Cu-Kα beam was used for the measurements of x...
examples 1-4
[0155]Magnetic force sorting (“raw material magnetic separation” in Table 1) to electrolytic manganese dioxide (contains 0.03 percent in mass Mg; TG diminution amount during 200° C.-400° C. heating: 3.0%) was carried out as indicated in Table 1, whereby using the magnetic force sorter described below which the ratio of the surface area of the magnet with respect to the magnetic force sorting distance in the up / down direction is adjusted to fall in the range of 800 mm2 / mm to 900 mm2 / mm, and introducing manganese raw material at an introduction speed of 1.0 kg / min.
[0156]So as to obtain the composition indicated in Table 1, lithium carbonate, electrolytic manganese dioxide, magnesium oxide and aluminum hydroxide, furthermore, with respect to the total weight thereof, 0.4 percent in weight of lithium borate (Li2B4O7) and water were mixed and stirred to prepare slurry with a solid content concentration of 25 percent in weight.
[0157]To the obtained slurry (10 kg raw material powder), an a...
example 5
[0166]Magnetic force sorting of electrolytic manganese dioxide (contains 0.03 percent in mass Mg; TG diminution amount during 200° C.-400° C. heating: 3.0%) was not carried out.
[0167]So as to obtain the composition indicated in Table 1, lithium carbonate, electrolytic manganese dioxide, magnesium oxide and aluminum hydroxide, furthermore, with respect to the total weight thereof, 0.4 percent in weight of lithium borate (Li2B4O7) and water were mixed and stirred to prepare a slurry with a solid content concentration of 25 percent in weight.
[0168]Thereafter, a sample was obtained similarly to Example 3.
[0169]When the obtained sample was analyzed by ICP by removing impurities such as SO4, it was determined that the composition was that shown in Table 1. The boron content was 0.1 percent in weight of the sample. In addition, the inter-atomic distance Li—O (“Li—O”), the crystallite size and the amount of magnetic substance of the obtained sample are indicated in Table 1, at the same time...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Magnetic field | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com