Rsv f protein compositions AMD methods for making same
a technology of protein composition and rsv, which is applied in the field of rsv f protein composition, can solve the problems of purification, homogeneous, and difficult to obtain immunogenic preparations, structure and refolding,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
RSV F Polypeptides
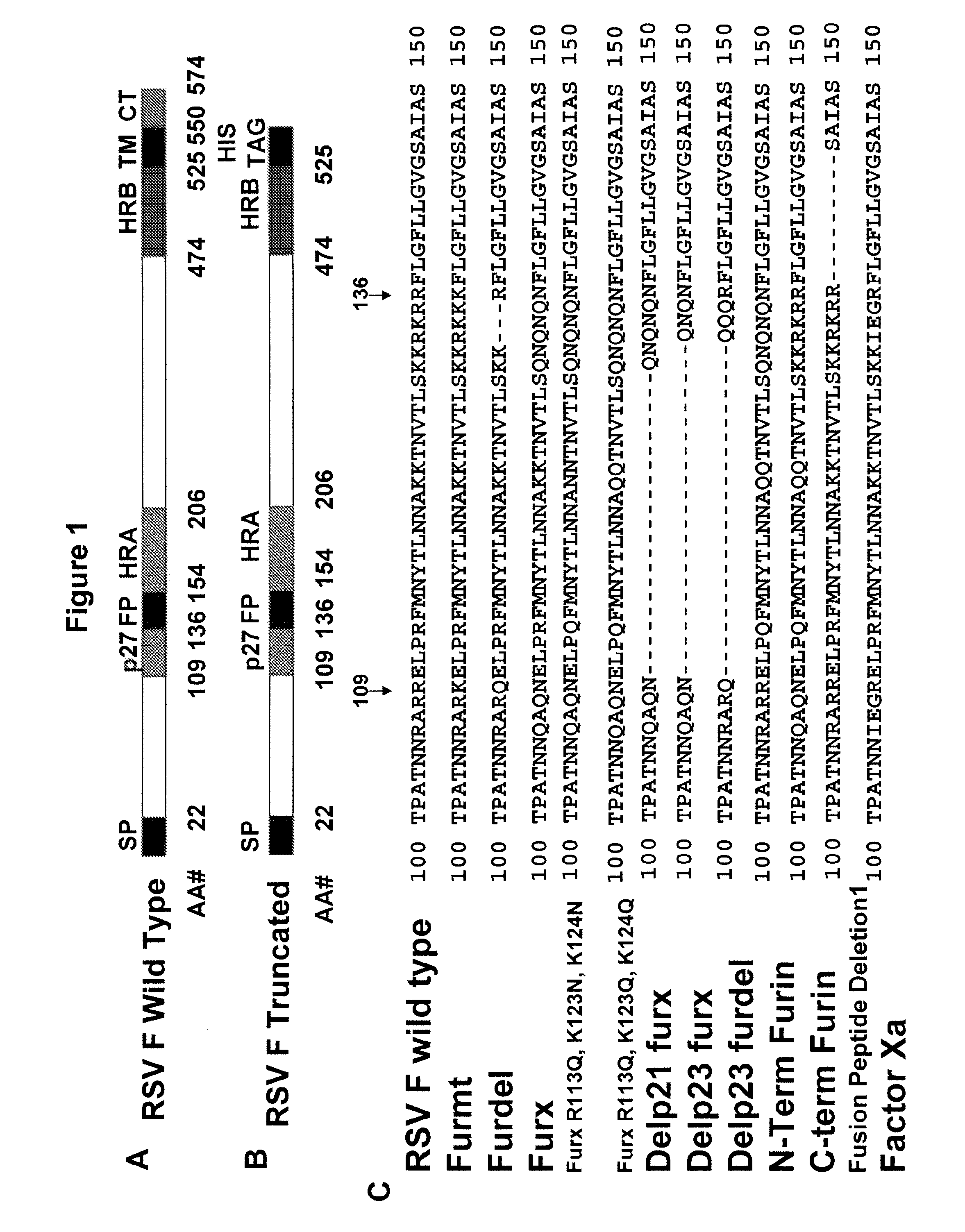
[0467]This example provides sequences of a number of examples of polypeptides (e.g., that contain signal sequences) and nucleic acid sequences that may be used to express RSV F polypeptides of the present invention. The presented amino acid sequences include the signal peptide and contain an optional C-terminal linker and His tag (GGSAGSGHHHHHH (SEQ ID NO:90)). When these polypeptides are produced in host cells, the polypeptide will usually be processed by the cell to remove the signal peptide and, as described herein, some of the polypeptides will be cleaved, for example at unmodified furin cleavage sites. The invention includes compositions that contain, all forms of the particular RSV F protein ecto-domain polypeptides disclosed herein, including mature forms, which lack the signal peptide, forms that may be cleaved into subunits that comprise F1 and F2, and forms that lack the optional C-terminal His tag. The following examples are merely illustrative of the sc...
example 2
Expression and Purification of RSV F Constructs
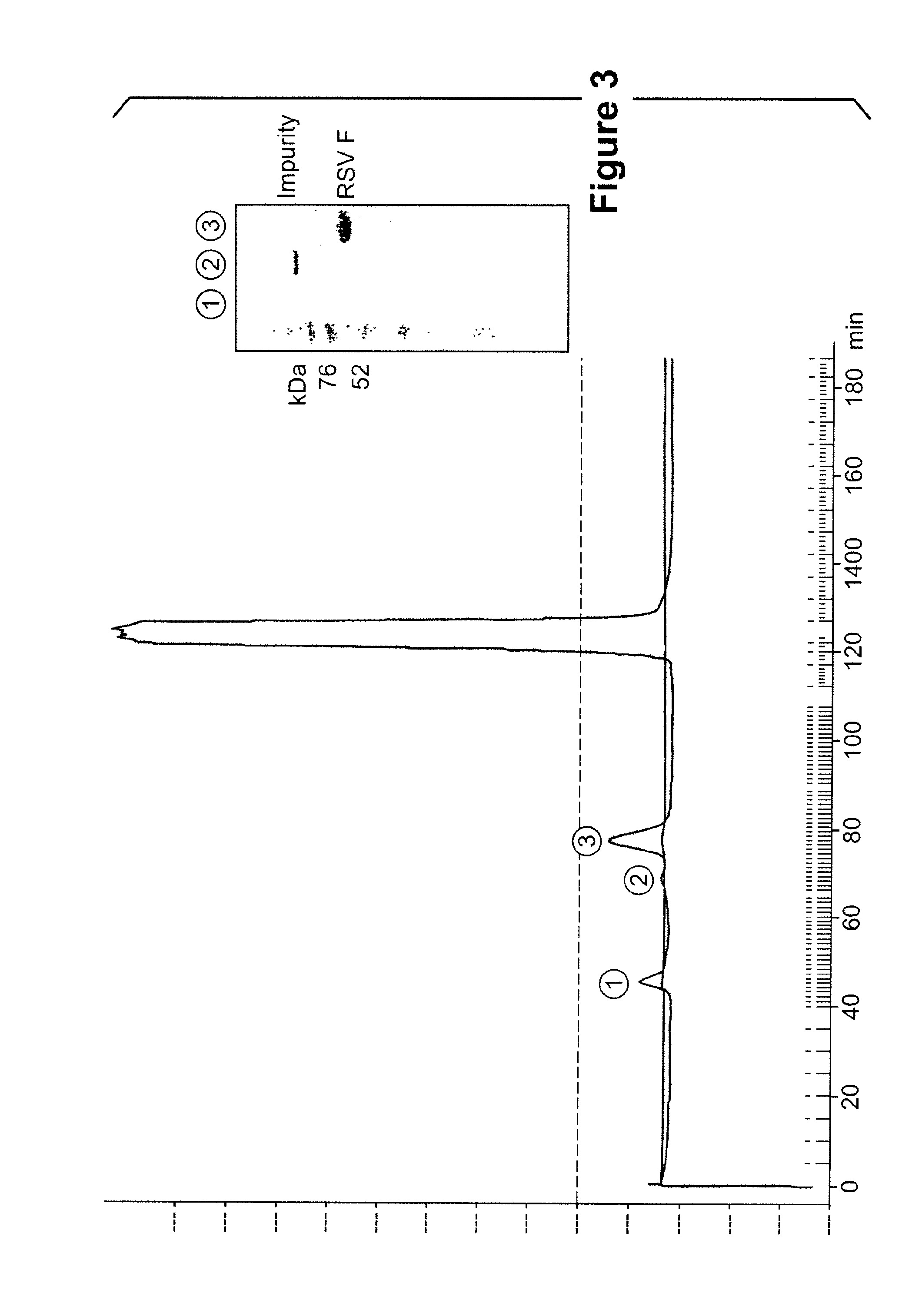
[0486]The RSV F ECTO and truncated constructs, lacking the transmembrane domain and cytoplasmic tail region with either wild-type furin cleavage sites or harboring knock-out mutations to the furin cleavage sites and with or without prefusion stabilization mutations, were cloned into a pFastBac baculovirus expression vector (Invitrogen). Several of these constructs contain a C-terminal flexible linker followed by a His6-tag sequence used for chelating purification. The production of high-titer baculovirus stocks were passaged in Sf9 insect cells. Proteins were expressed by infecting either Sf9, Tn5 or High Five insect cells with the required baculovirus and harvesting the media supernatant two or three days post infection, monitored by western blot using an anti-RSV F or anti-6HIS antibody.
[0487]Large scale expression media was concentrated / purified by one of two general strategies for eliminating the deleterious Effect of the ferritin p...
example 3
Detection of Pre-Fusion and Post-Fusion RSV F
[0496]A number of methods are available to determine the conformation of the RSV F protein to assay whether a modification to the RSV F polypeptide or added molecule disfavors the post-fusion conformation. Examples include liposome association, conformation specific monoclonal antibodies (including as used in FACS, ELISA, etc.), electron microscopy, differential protease sensitivity between the conformations, gel filtration chromatography, analytical ultracentrifugation, dynamic light scattering, deuterium exchange NMR experiments, mass spectroscopy, circular dichroism spectroscopy, isothermal titration calorimetry, tryptophan spectroscopy, and X-ray crystallography.
Liposome Association
[0497]Liposome association may be used to assay the conformation of the RSV F protein. Soluble forms of the RSV F protein in the pre-fusion conformation will not associate with liposomes while the post-fusion conformation will associate with liposomes.
[0498...
PUM
| Property | Measurement | Unit |
|---|---|---|
| immunogenic composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com