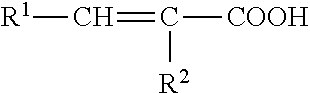Functionalized monomers
a functionalized monomer and monomer technology, applied in the direction of fatty acid chemical modification, detergent compounding agent, fuel, etc., can solve the problems of poor solvency, poor thermal stability, and insufficient lubricants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
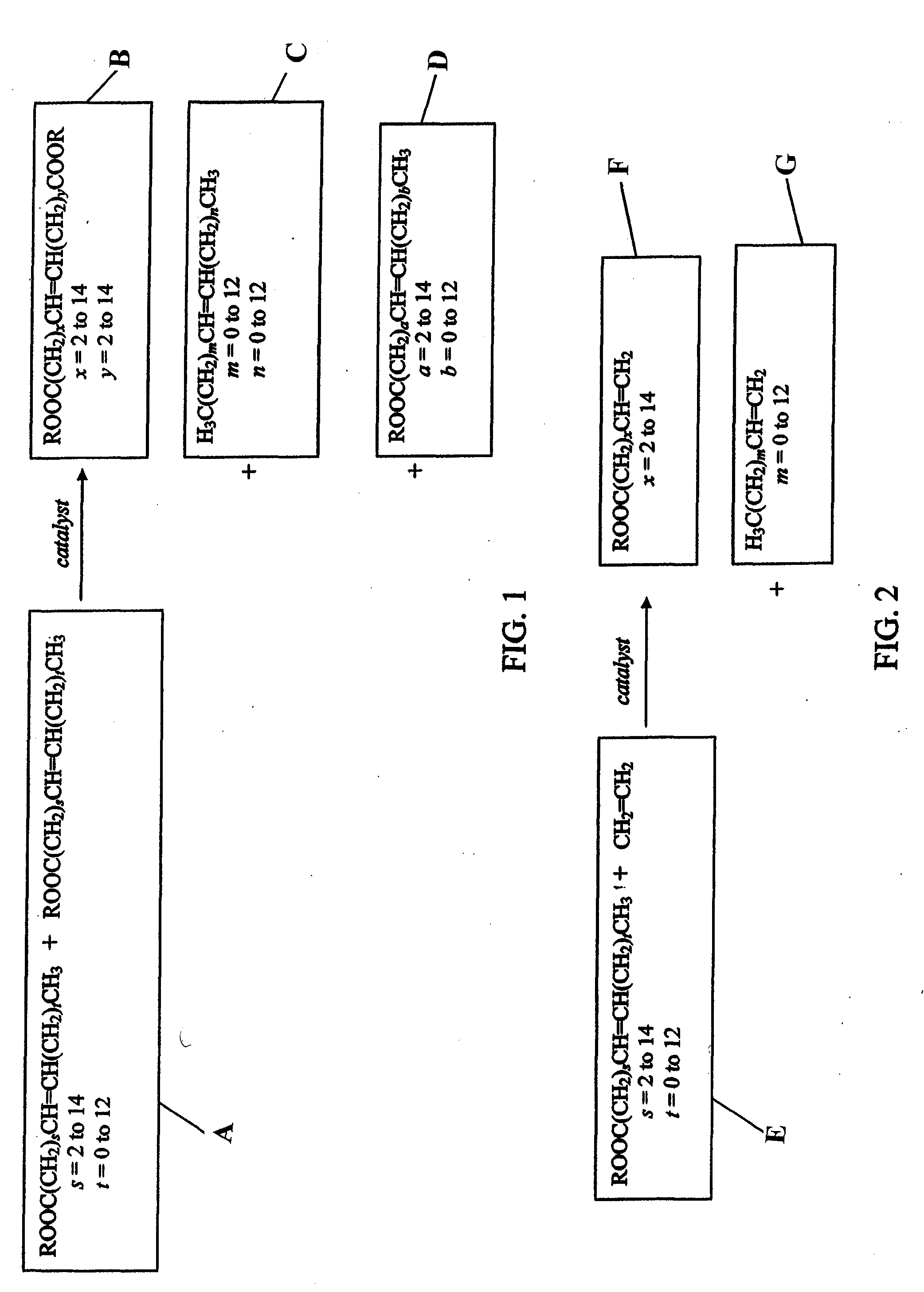
[0374]The following example discloses the oligomerization of 1-decene with methyl 9-decenoate (which may be referred to as 9-DAME or 9-DAMe) in the presence of di-t-amyl peroxide. The reaction proceeds according to the following equation using the reagents shown in the table below.
1-decene9-DAMEdi-t-amyl peroxideFW140.27184.28174.28equivalents10.001.00*mol0.80460.08050.1408**g112.8614.8324.54d (g / ml)0.740.900.82ml152.316.530.00*Note:9-DAME is 9.09 mol % of the total monomer mixture.**1.75X “the original” peroxide loading
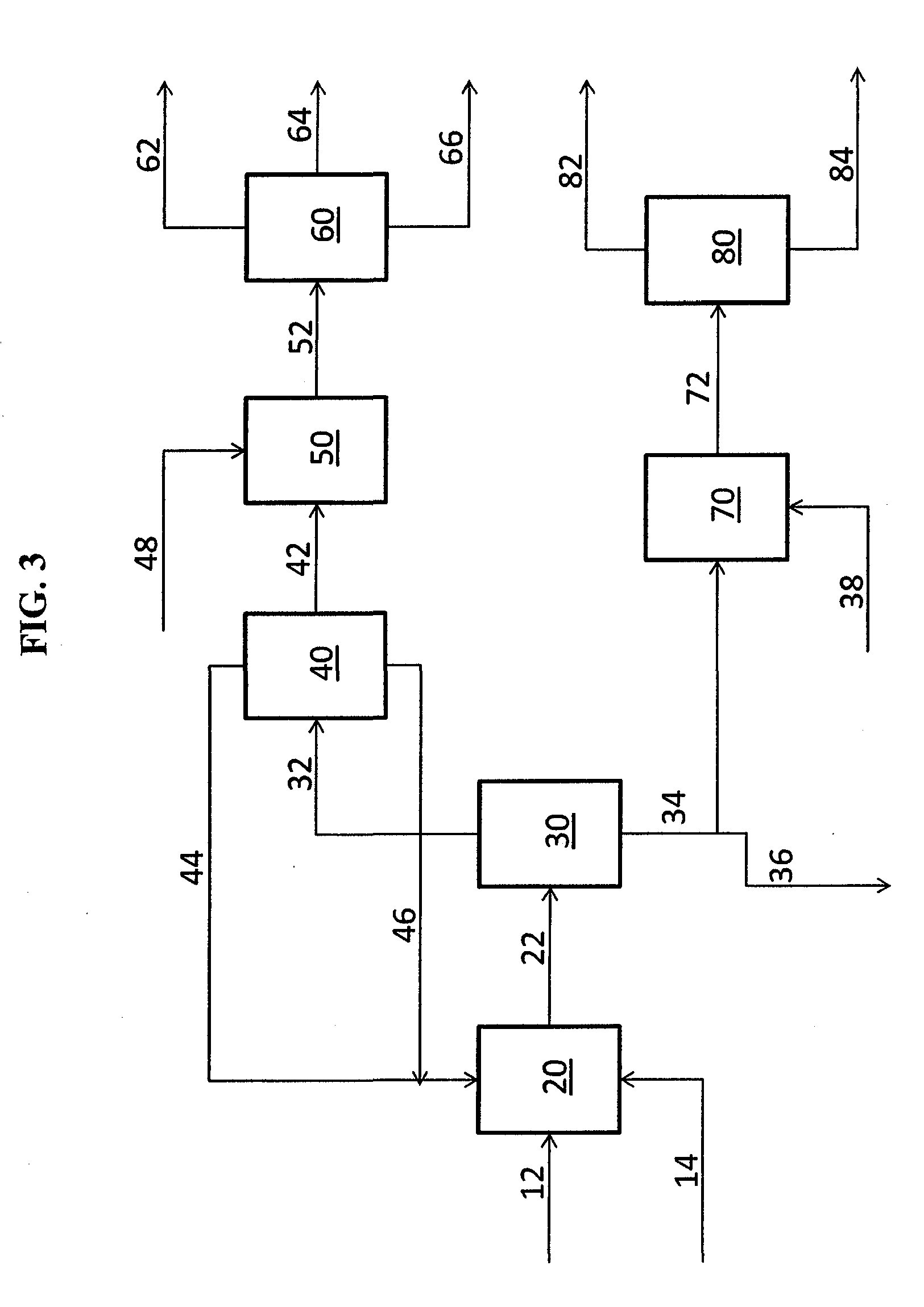
The following process steps are followed:
[0375]A 250 ml 3-necked round-bottomed flask, equipped with a magnetic stirrer, inert gas inlet / outlet, rubber septum, thermocouple controlled heating mantle, and Dean-Stark trap with condenser, is charged with 1-decene and 9-DAME. The monomer mixture is sparged with inert gas for 1 hour to overnight.
[0376]After sparging, the monomer mixture is heated to 150° C. After reaching reaction temperature, the first 1 / 10 volume portio...
example 2
[0398]The following example discloses a process for the maleinization of methyl 9-decenoate (9-DAME). 9-DAME is reacted with maleic anhydride in an ene reaction to introduce one to two succinic groups per ester. Maleic anhydride is known to participate in ene reactions; this is due to the combined electron-withdrawing power of the two carbonyl groups adjacent to the double bond. The mechanism is believed to involve an allylic shift of one double bond by transferring the allylic hydrogen to the enophile and generating a new bond. The reaction may be promoted by heat and / or Lewis acids. In this reaction no catalyst is used.
[0399]2532.9 g (13.74 mol) 9-DAME and 1684.8 g (17.18 mol, 1.25 eq) of maleic anhydride are added into a 5 L three neck flask equipped with a large egg-shaped magnetic stirbar. The flask is then set into a heating mantel (two piece soft heating mantel with zipper). Two condensers are attached, one for incoming nitrogen gas and the other one for the outgoing gas flow...
example 3
[0400]The following example discloses a process for the maleinization of 9-octadecenoic acid dimethyl ester (ODDA FAME). After filtration over silica gel 1277.7 g (3.75 mol) of ODDA FAME are reacted with 441.5 g (4.50 mol, 1.2 eq) of maleic anhydride. After a reaction time of 6 hours at 200° C. the product is stripped in high vacuum to remove unreacted maleic anhydride. The product is a viscos, brown oil, which is transparent in smaller volumes. NMR shows no residual free maleic anhydride in the stripped product. The AV for the product is 233.8. The conversion is 91.4%. The KV (cSt) at 100° C. is 17.32. The product may be represented by the following formula:
Oxidized Derivative
[0401]The functionalized monomer or functionalized polymer of the invention may be reacted with one or more oxidizing agents. This may result in the formation of one or more oxidized derivatives which may be in the form of one or more epoxides. These may be referred to as polyfunctionalized monomers or polymer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| acid | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com