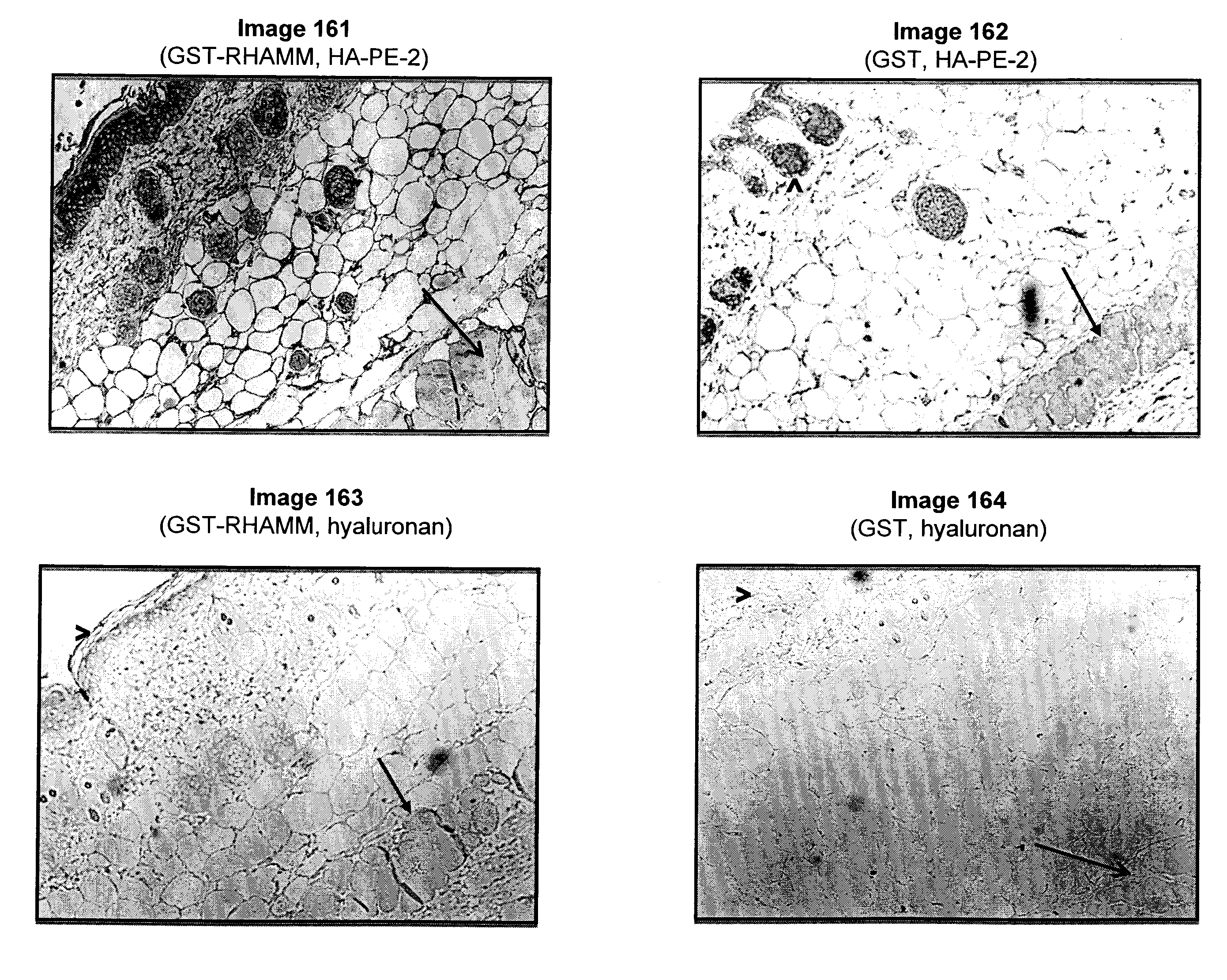
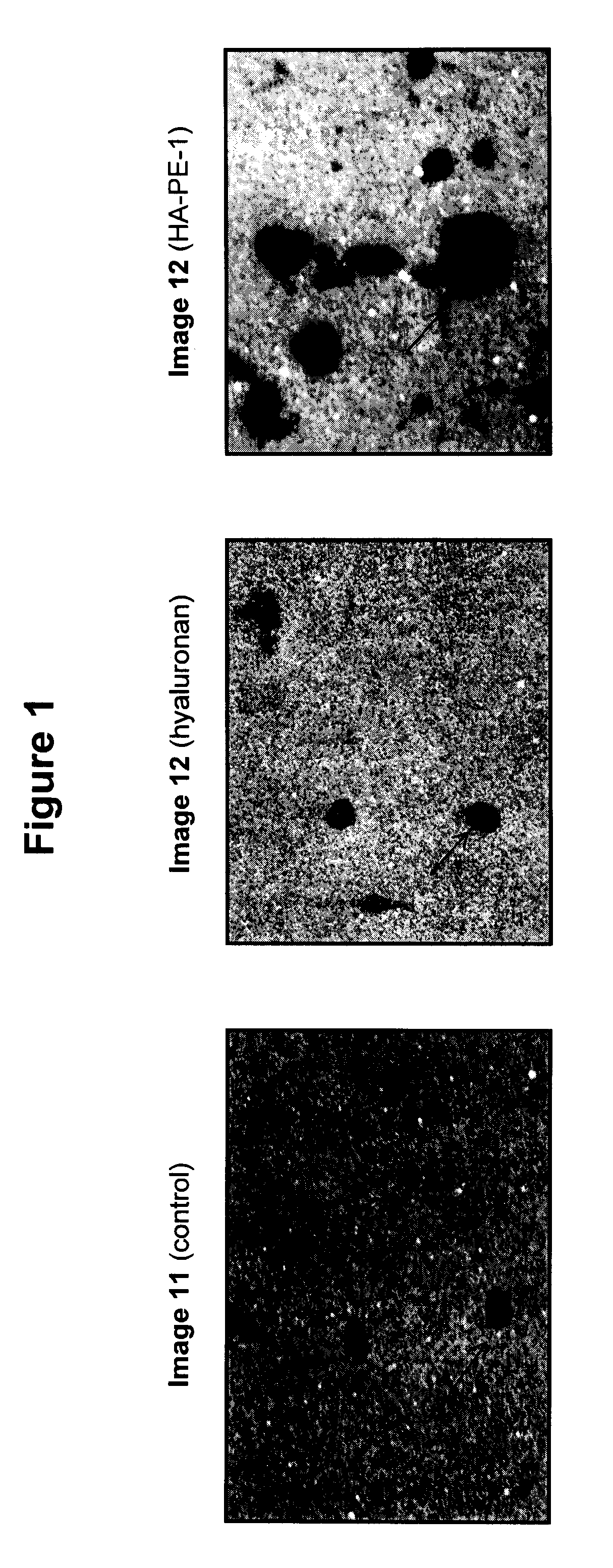
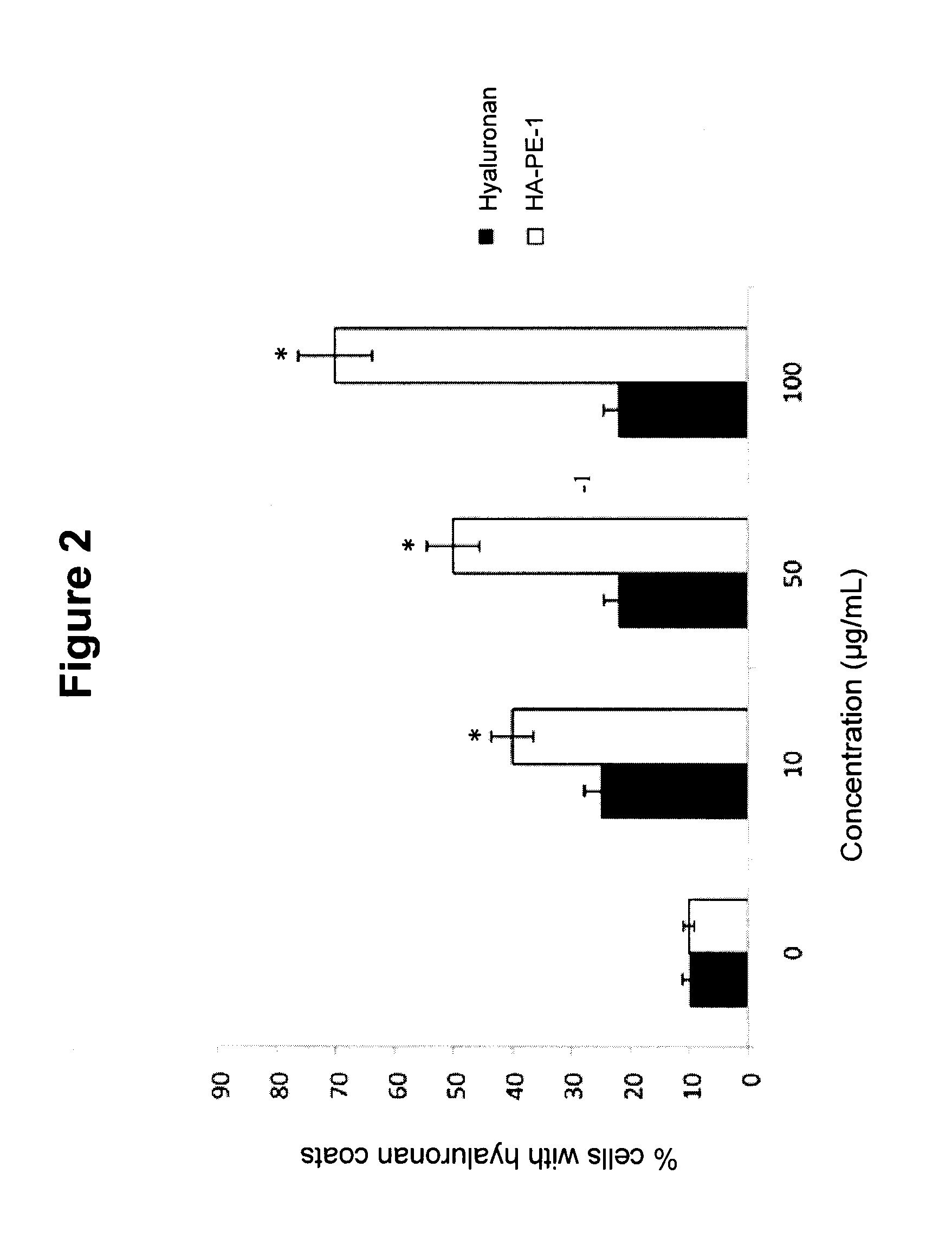
Topically administered, skin-penetrating glycosaminoglycan formulations suitable for use in cosmetic and pharmaceutical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a Non-Covalently Linked Hyaluronan-Phosphatidylethanolamine Conjugate (HA+PE)
[0157]An associated (non-covalently linked) hyaluronan-phosphatidylethanolamine complex (HA+PE) was prepared by mixing an aqueous hyaluronan solution (78.5 mL, 12 mg / mL; Baxyl®, Cogent Solutions Group, Lexington, Ky., USA) with soybean lecithin (78.5 mL; Soya Lecithin GT Non-GM IP containing 13% phosphatidylethanolamine; Imperial-Oel-Import) in isopropanol (10 mL) (to promote mixing) at room temperature for 30 minutes in a blender. The mixture was then incubated at 4° C. for 48 hr before being used in the preparation of a topical cream as described for the linked compositions of the invention in Example 4.
[0158]The lecithin used, in this example and the other examples described herein, contained 15% phosphatidylcholine, 13% phosphatidylethanolamine, 10% phosphatidylinositol, 19% other lipids, 5% carbohydrates, and 38% soybean oil. These phospholipids contain primarily C14-C22 fatty acids as ...
example 2
Preparation of a Linked Hyaluronan-Phosphatidylethanolamine Conjugate (HA-PE-1)
[0161]A covalently linked hyaluronan-phosphatidylethanolamine conjugate (HA-PE-1) was prepared by pre-mixing a hyaluronan solution (5 mL, 10 mg / mL de-ionized water, 50 mg; ˜350 kDa (Life Core, Minn., USA), ˜1.3×10−4 mol —CO2H groups) with phosphatidylethanolamine (PE) (250 mg; Sigma-Aldrich®, cat no. 60648; 500 mg assayed at ˜50%) with rapid stirring via hand blender. Prior to addition of the phosphatidylethanolamine to the hyaluronan, it was first dissolved in chloroform (0.5 mL), which was then evaporated off, replaced with isopropanol (0.5 mL), and brought into suspension with a hand vibrating probe. 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) (1.2 mg, 7.7×10−6 mol; 10 μL of a freshly prepared stock solution containing 120 mg EDC dissolved in de-ionized water (1 mL)) was added and thoroughly mixed for 30 minutes, then left at room temperature for 2 hours.
[0162]HA-PE-1 was used in the cellular a...
example 3
Preparation of a Linked Hyaluronan-Phosphatidylethanolamine Conjugate (HA-PE-2)
[0164]In addition to the use of pure phosphatidylethanolamine, such as in Example 2, mixtures of lipids containing phosphatidyl ethanolamine may also be used. For example, liquid soy lecithin (Soya Lecithin GT Non-GM IP, Imperial-Oel-Import) contains approximately 15% phosphatidylcholines, 13% phosphatidylethanolamines, 10% phosphatidylinositols, 19% other phospholipids and lipids, 5% carbohydrates, and 38% soybean oil. This is approximately 330 mg phosphatidylethanolamines (the only lipid expected to react in large amounts using EDC as a linking agent) per tablespoon (14.79 mL).
[0165]Unrefined liquid soy lecithin (78.5 mL; Soya Lecithin GT Non-GM IP, Imperial-Oel-Import) was mixed with hyaluronan (78.5 mL, 12 mg / mL, 942 mg, ˜2.5×10−3 mol —CO2H groups; 500-2,500 kDa, polydisperse Baxyl HA, Cogent Solutions Group, Lexington, Key.) and isopropanol (10 mL) with rapid stirring via hand blender for 10-15 minut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com