Gaseous nitric oxide-sequestering products and processes of preparing same
a technology of gaseous nitric oxide and products, applied in the field of medical products, can solve the problems of increasing the risk of infection, and constant risk to any healthy person, so as to reduce the pressure in the chamber
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
NO-Impregnated Silicone Catheters
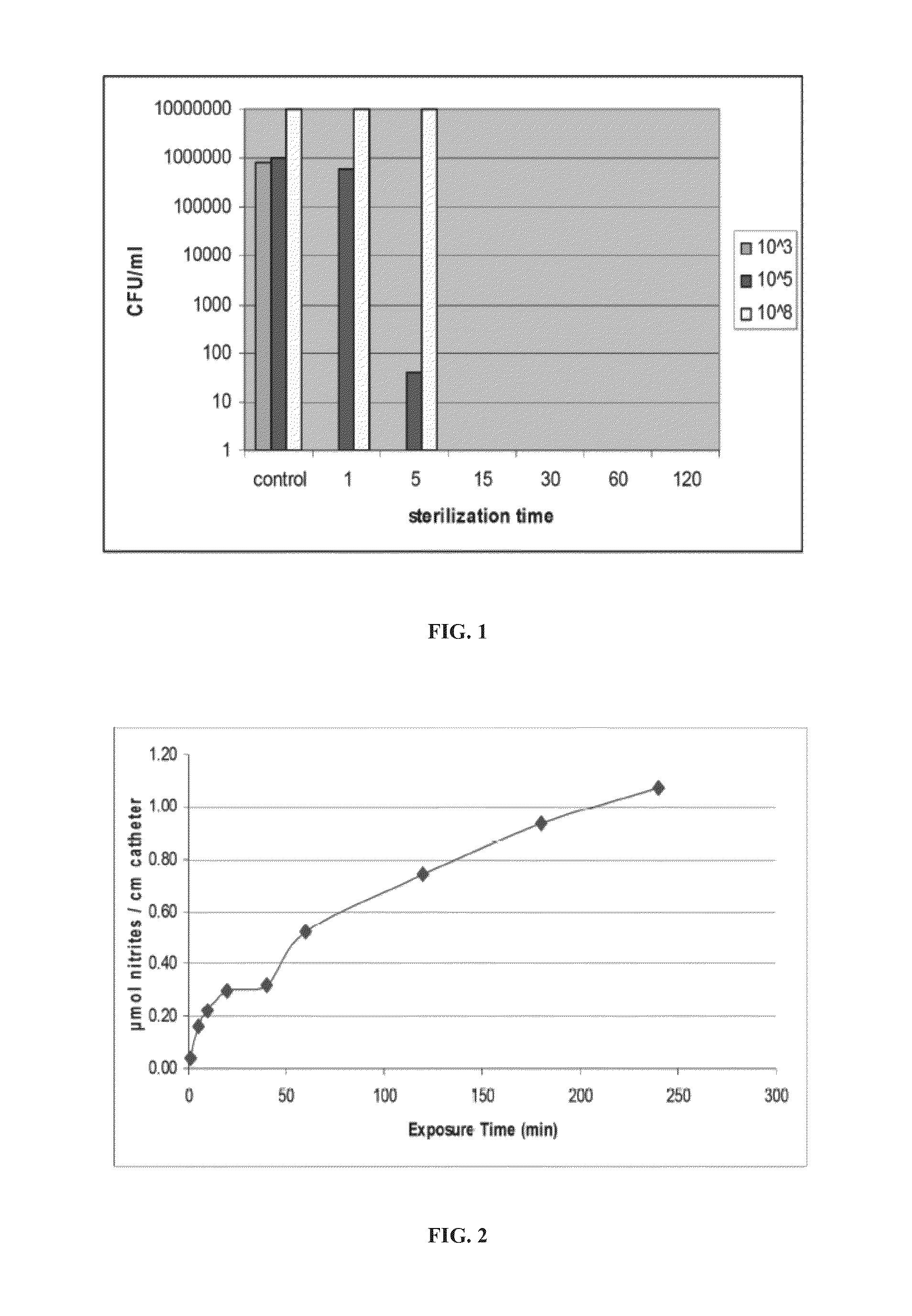
[0442]Six-mm diameter Folysil® silicone Foley catheters, (catalog no. AA6118; Coloplast® Corp. Minneapolis, Minn., USA) (Folysil® and Coloplast® are registered trademarks of Coloplast A / S, Humlebaek, Denmark) were aseptically cut into 2-cm sections and inoculated by immersion in 2 ml of one of 103 CFU / ml, 105CFU / ml, or 108 CFU / ml E. coli. inocula for 30 minutes. Inoculated catheter sections were subsequently dosed with 20,000 parts per million of nitric oxide (at a flow rate of 30 cc / minutes) during 1, 5, 15, 30, 60 or 120 minutes, using the general procedure I described hereinabove. Control catheter sections were not impregnated with nitric oxide, but were stored in sterile sealed vials.
[0443]The nitric oxide-treated and untreated catheter segments were then immersed separately in 2 ml media and then incubated for 8 hours at 37° C. Aliquots of the incubation media were plated and numbers of CFU counted. Bacterial loads were calculated as CFU per ml....
example 2
NO-Impregnated Silicone Catheters
[0447]A commercially-available catheter, such as a six-mm diameter Folysil® silicone
[0448]Foley catheters, (catalog no. AA6118; Coloplast® Corp. Minneapolis, Minn., USA) is aseptically cut into 2-cm sections and inoculated by immersion in 2 ml of one of 103 CFU / ml, 105 CFU / ml, or 108 CFU / ml E. coli. inocula for 30 minutes. Inoculated catheter sections are subsequently dosed with nitric oxide using the general procedure II described hereinabove. Control catheter sections are not impregnated with nitric oxide, but are stored in sterile sealed vials.
[0449]The nitric oxide-treated and untreated catheter segments are then immersed separately in 2 ml media and incubated for 8 hours at 37° C. Aliquots of the incubation media are plated and numbers of CFU counted. Bacterial loads are calculated as CFU per ml.
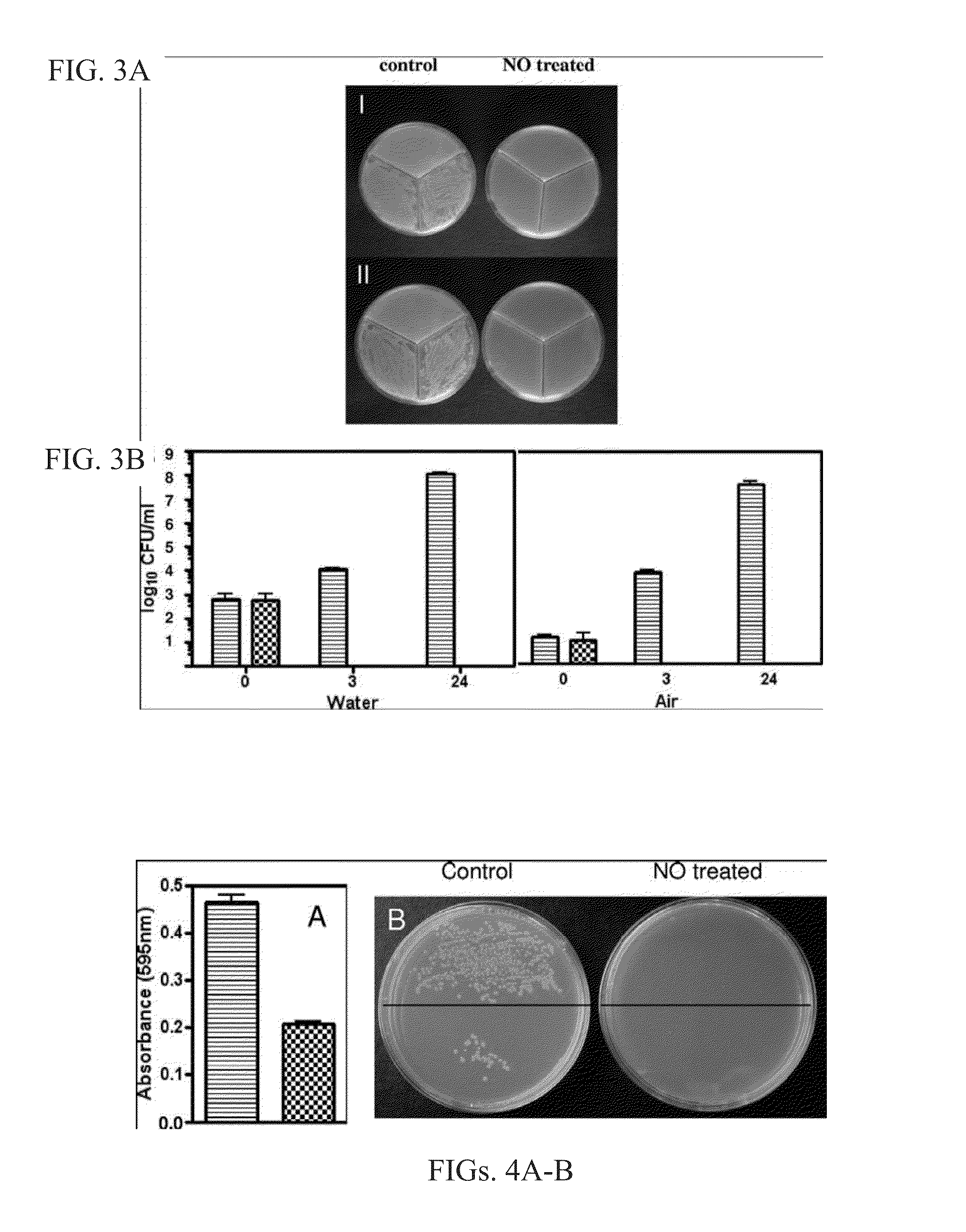
[0450]Catheters impregnated with NO using general procedure II are found to eradicate bacteria at all of the tested bacteria concentrations. Sterilizati...
example 3
Nitric Oxide Loading in NO-Impregnated Catheters
[0451]Six-mm diameter Folysil® silicone Foley catheters were aseptically cut into 2-cm sections and exposed to nitric oxide for 1, 5, 10, 20, 40, 60, 120, 180 or 240 minutes, using general procedure I as described hereinabove. The samples were then immersed in doubly-distilled water and after 1 hour were assayed for nitrates and nitrites, as described hereinabove. All calculations were done per 1 cm of catheter.
[0452]FIG. 2 presents a plot of nitrite in μmol per cm of catheter relative to dosing time.
[0453]Table 2 presents the raw data of release of nitrates and nitrites from nitric oxide dosed catheter sample over varying dose times, wherein “Abs.” denotes absorbance, and “Abs average” denotes the average absorbance measured from duplicate sampling.
TABLE 2NO2conc.μmol NO2NOTimeAbsNO2inμmol / ppm / (min)Abs.Abs.average(μM)solutioncmcm10.0660.0690.0725.960.080.040.3950.2750.2740.27105.580.320.161.58100.3980.3640.38146.540.440.222.19200.4910...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| time period | aaaaa | aaaaa |
| humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com