Method for Inhibiting HIV Replication in Mammal and Human Cells
a technology of human cells and hiv, applied in the field of biomedicine, can solve the problems of poor adherence to treatment by patients, limited therapeutic options for patients receiving combined anti-hiv therapy, and inability to completely respond to treatment, so as to avoid viral resistance, reduce the probability of occurrence, and inhibit the effect of high inhibition capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
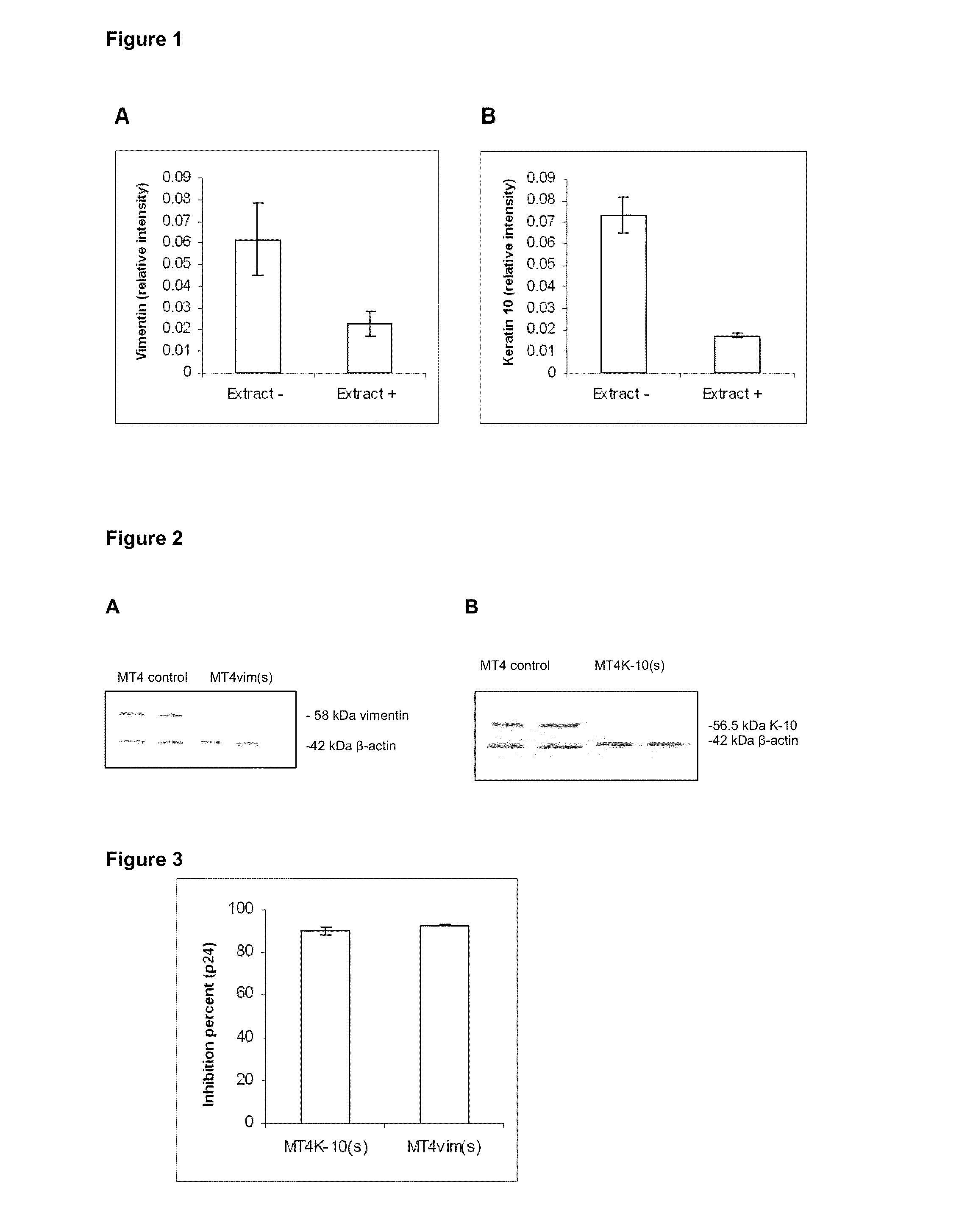
Comparative Proteomics of MT4 Cells Treated with a Leukocyte Extract Showing Anti-HIV Activity
[0125]The MT4 cell line was treated with a leukocyte extract showing anti-HIV activity (Fernandez-Ortega C; Dubed M; Ruibal I; Vilarrubia O L; Menéndez JC; Navea L et al. 1996, Biotherapy 9: 33-40) and the resulting protein expression profile was compared to a control of untreated cells. The cells were lysed and centrifuged at 12 000 rpm for 20 min. The supernatant was collected and the pellet was subjected to a second lysis procedure. After a second centrifugation step under the same conditions, the second supernatant was collected together with the first one, being further delipidated with ethyl alcohol and alkylated with polyacrylamide. Afterwards, the deoxyribonucleic acid (DNA) was precipitated and a bidimensional electrophoresis of the sample was carried out by using a 12.5 to 3% Tris-Tricine polyacrylamide gel at 4° C.
[0126]The images of analytical gels were analyzed by using the Mel...
example 2
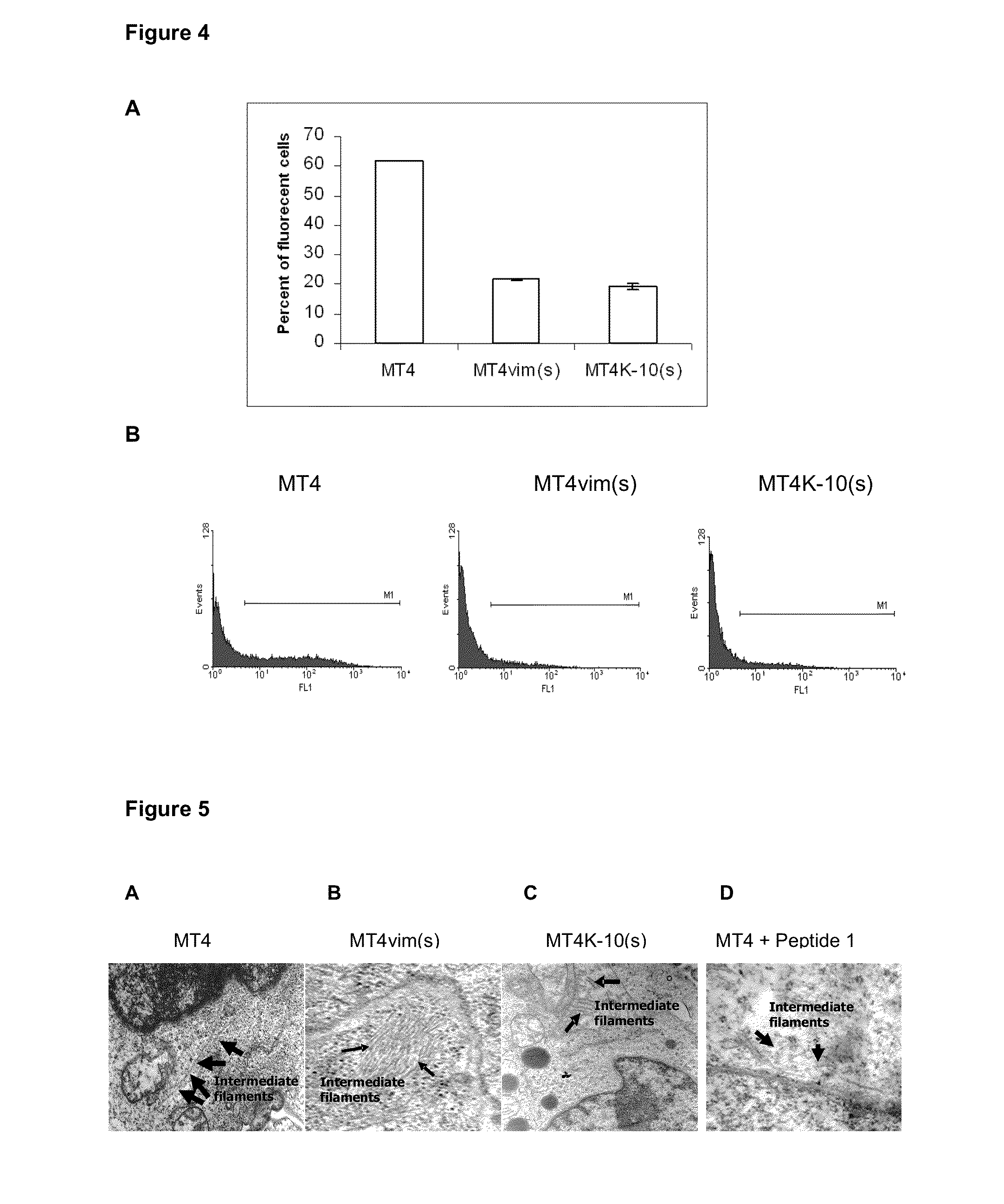
Interfering RNA Against Vimentin and Keratin-10 Inhibits HIV Infection
[0128]The MT4 cell line was transduced by using the pLenti-shRNAvim or pLenti-shRNAK-10 lentiviral vectors, which bear a sequence encoding a RNA hairpin which silences the expression of the vimentin and keratin proteins, respectively. These lentiviral vectors were assembled by packaging in the 293T cell line transduced with four plasmids. The said plasmids were pLP1, pLP2, pLP / VSVG and p-shRNA, this last specific for either vimentin or keratin-10. The pLP1 vector codes for the gene products of the gag / pol sequences of HIV-1. The pLP / VSVG codes for the surface protein of the vesicular stomatitis virus and the p-shRNA contains the genome of the lentiviral vector which bears the sequences coding for the vimentin- or keratin-10-specific RNA hairpins (Ui-Tei K, Naito Y, Takahashi F, Haraguchi T et al., 2004 Nucleic Acids Research 32: 936-948; Santa Cruz Biotechnology). All the plasmids were amplified in the Escherichia...
example 3
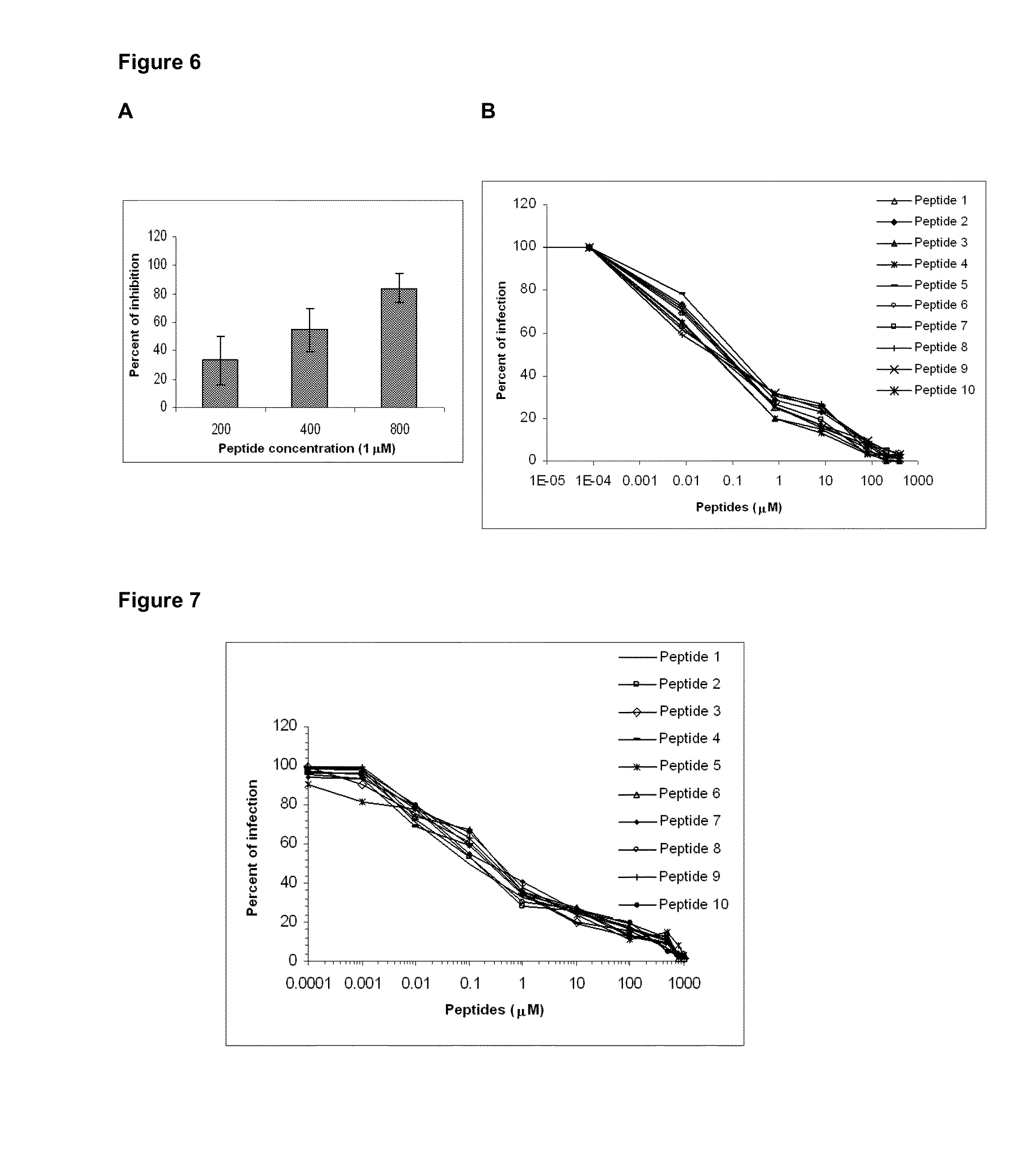
Changes in the Structure of Intermediate Filaments in MT4 Cells
[0132]Firstly, MT4vim(s), MT4K-10(s) and MT4 cells were fixed in 3.2% glutaraldehyde for 1 h at 4° C. and then fixed in 2% osmium tetroxide for 1 h at 4° C. They were subsequently washed with 0.1 M phosphate buffered saline (PBS), pH 7.2, and dehydrated at increasing ethanol concentrations (30, 50, 70 and 100%) for 10 min each at 4° C. Inclusion was carried out and ultrathin 40-50 nm-width sections were taken in an ultramicrotome (NOVA, LKB), which were placed on 400-orifices nickel trays. Once the ultrathin cuts were taken and placed on the trays, they were contrasted with saturated uranyl acetate and lead citrate, and further examined under a JEOL JEM 2000 EX (JEOL) microscope. Five microphotographs were analyzed at different magnifications. MT4 cell intact IFs are shown in FIG. 5A, meanwhile these structures appeared shortened in MT4vim(s) MT4K-10(s) cells (FIGS. 5B and C, respectively). Section D shows that effect in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com