Method for Preparing 3,6-Anhydro-L-Galactose, And Use Thereof
a technology of anhydrolgalactose and a method of preparing it, which is applied in the field of 3, 6anhydrolgalactose, can solve the problems of low yield, easy degradation to excessive extent, and low yield of desired product, and achieves the effect of minimizing over-degradation effects and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Hydrolysis of Agarose Using Acetic Acid
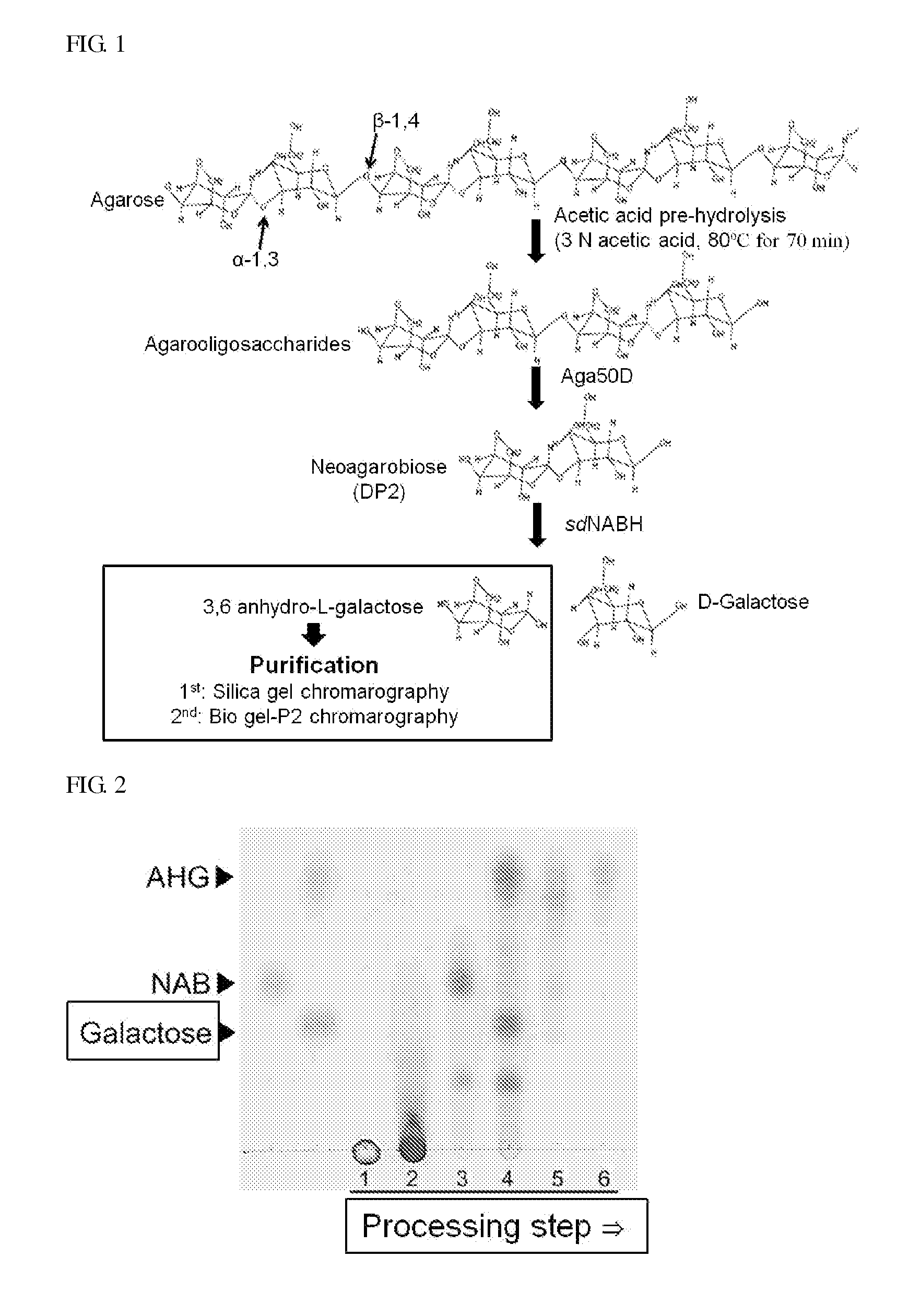
[0106]Agarose that is a representative polysaccharide constituting a sea alga was hydrolyzed with acetic acid. 5% (w / v) of agarose was reacted with 3M acetic acid at 80° C. for 70 minutes and dried to remove acetic acid. Also, acetic acid which remained after drying through a washing procedure using ethanol, and over-degradation products that were able to be produced upon hydrolysis were removed to produce an agarooligosaccharide in the form of a pure powder (FIG. 2).
example 2
Production of 3,6-Anhydro-L-Galactose Using Aga50D and sdNABH
[0107]To degrade the acid hydrolysate produced in Example 1 into monosaccharides, D-galactose and 3,6-anhydro-L-galactose, the acid hydrolysate was reacted with an exo-type disaccharide-producing enzyme, Aga50D, (see Korean Patent Publication No. 2010-0040438) to produce neoagarobiose as a reaction product.
[0108]When the reaction with Aga50D was completed, the Aga50D reaction product was reacted with an sdNABH enzyme (see Korean Patent Publication No. 2010-0108241) in order to produce the monosaccharides, D-galactose and 3,6-anhydro-L-galactose, from the neoagarobiose. The enzymatic reaction conditions were as follows: 5% (w / v) of agarooligosaccharide was dissolved in 100 mL of a 50 mM Tris-HCl buffer solution (pH 7.4), and reacted at 30° C. and 150 rpm for 3 days. The amounts of the enzymes used upon the enzymatic reaction were 10 mg and 2.5 mg for Aga50D and sdNABH, respectively (FIG. 2).
example 3
Separation and Purification Using Silica Gel Chromatography and Biogel P2 Chromatography
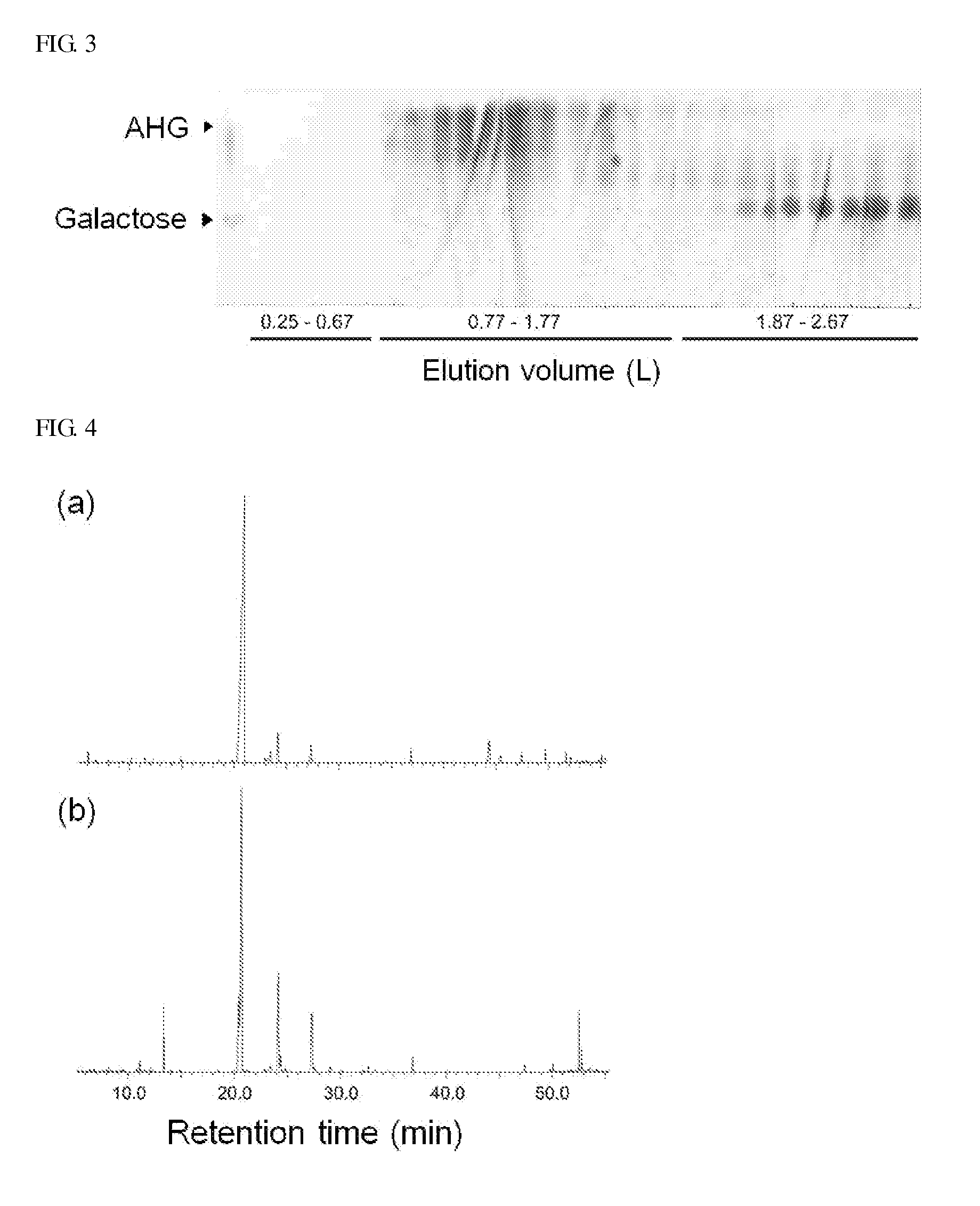
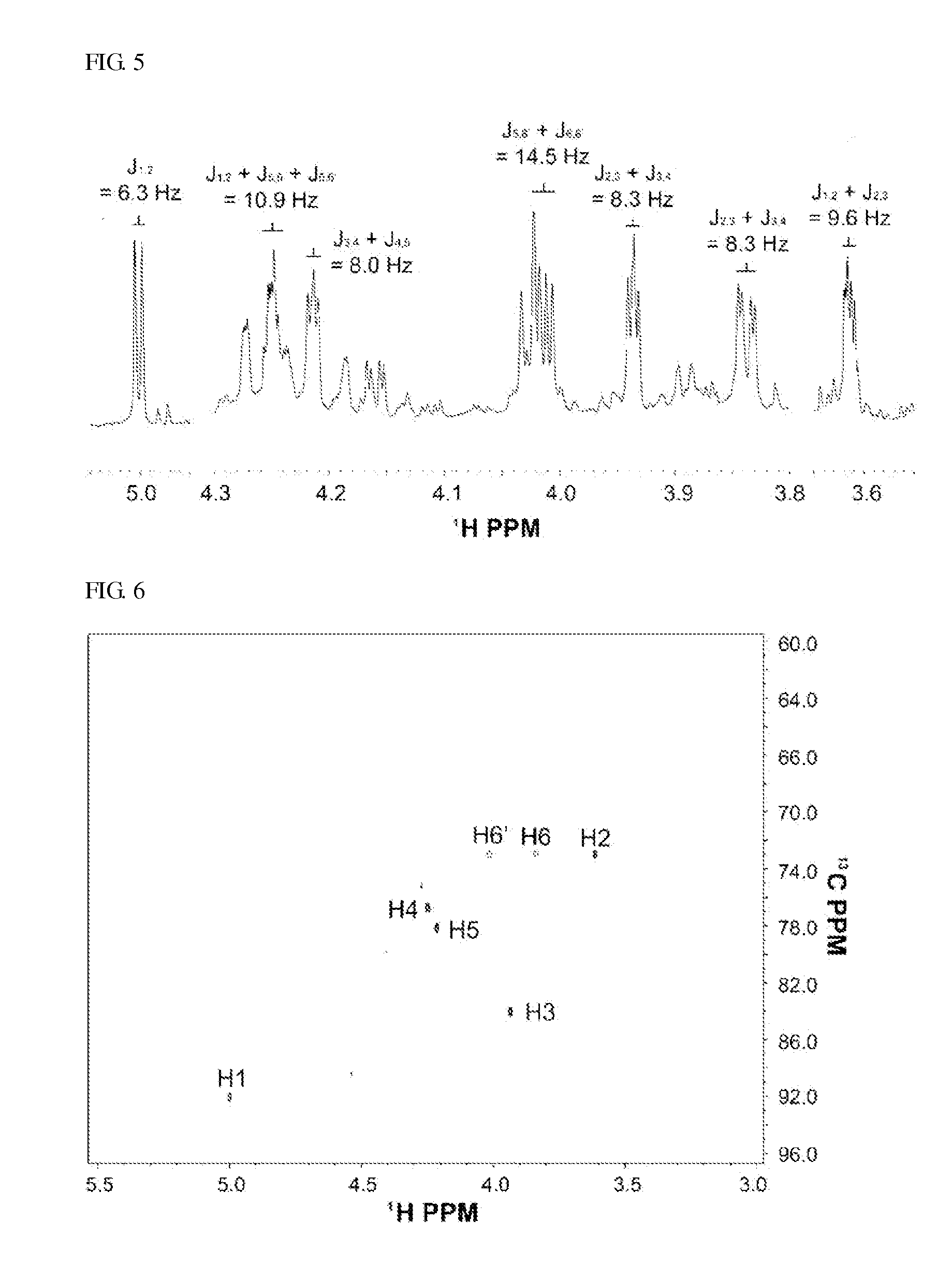
[0109]Chromatography was performed to separate and purify only 3,6-anhydro-L-galactose from the reaction products produced in Examples 1 and 2. The reaction products were adsorbed onto celite to form a sample in the form of powder, and subjected to silica gel chromatography that was adsorption chromatography. A solvent obtained by mixing chloroform, methanol, and water at a ratio of 78:20:2 (v / v / v) was used as a mobile phase, and the total volume of the solvent as the mobile phase was 3 L. The volume of one fraction was 20 mL, and the sample composed of a total of 150 fractions was analyzed through TLC. Among these, the fractions containing 3,6-anhydro-L-galactose were collected. Since the biogel P2 chromatography to be further performed separated materials according to the molecular weights, the fractions containing D-galactose having a molecular weight similar to 3,6-anhydro-L-galactose among t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com