Source of phosphate for agriculture and the food industry
a technology of phosphate and agriculture, applied in the field of phosphate sources for agriculture and the food industry, can solve the problems of soil permanent contamination, serious environmental damage,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
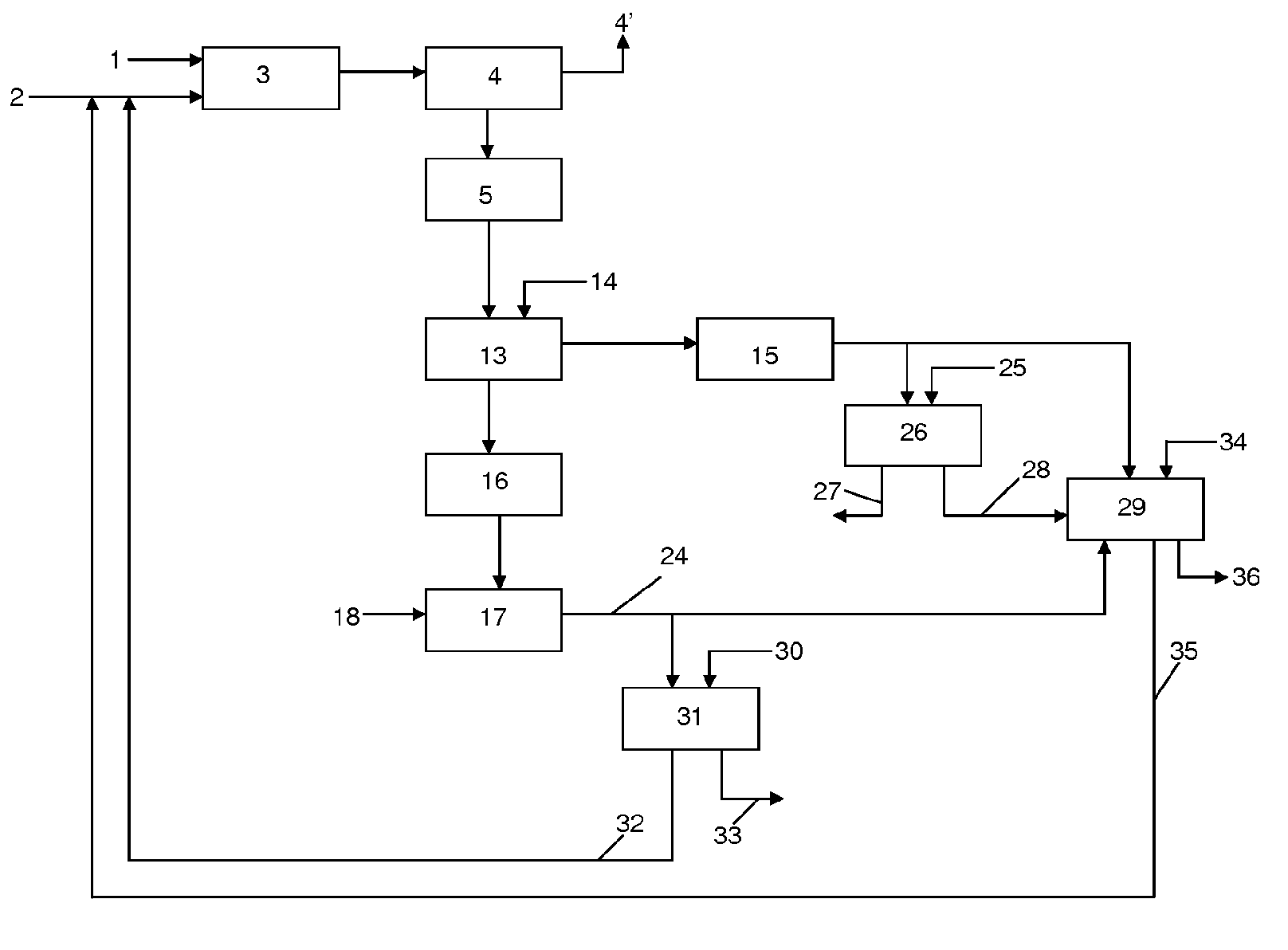
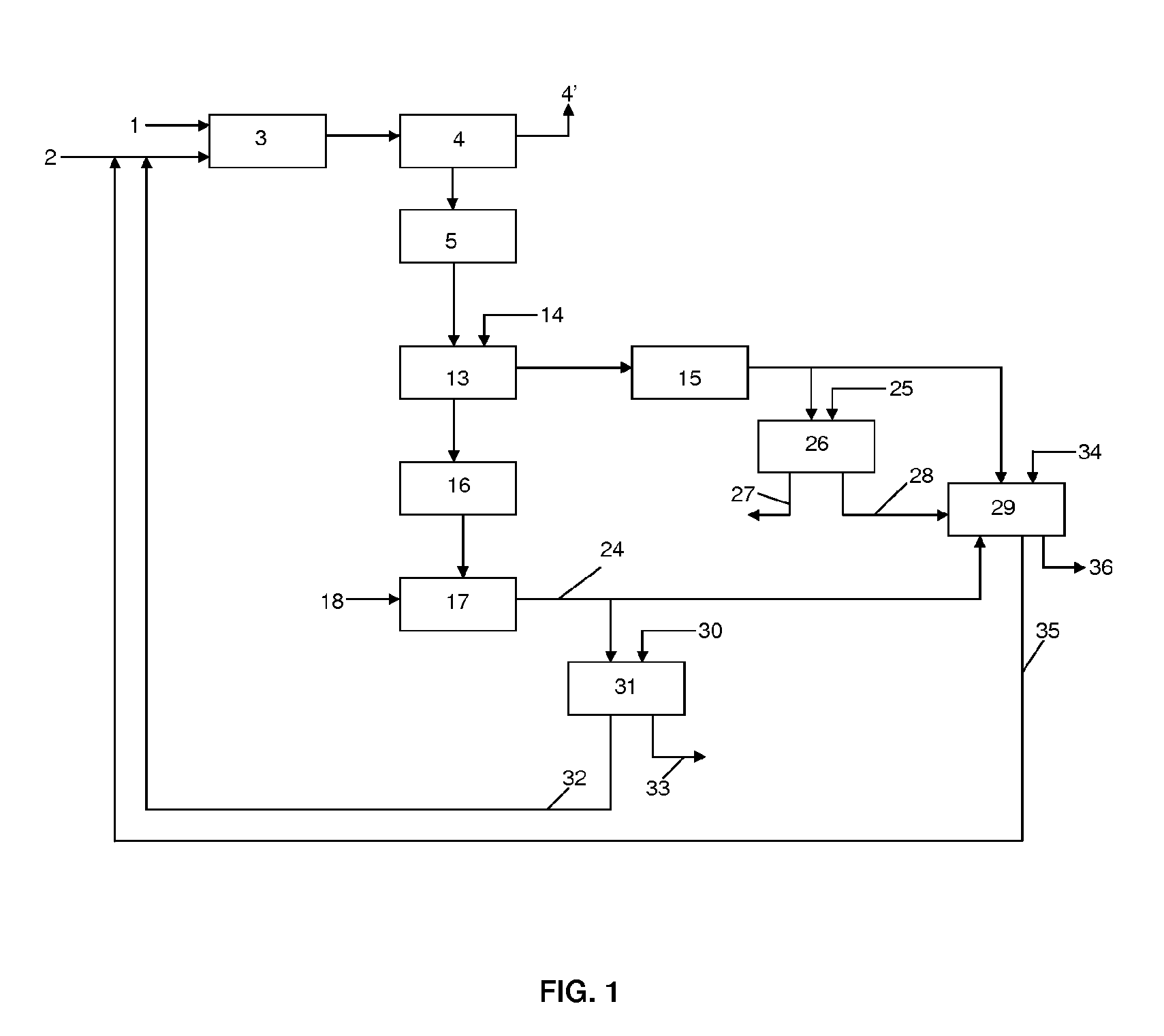
[0157]Phosphate ore from North Africa with a P2O5 content of 29% by weight is used. The attack of the ore is performed using an aqueous solution having HCl content by weight of 12% at a temperature of 60° C. The quantity of HCl added is determined by a molar ratio of the HCl added to the ore and the Ca present in it. The attack of the ore is performed at an HCl / Ca ratio of 1.8. The residence time in the attack reactor is 15 minutes. The attack liquor is then transferred to a filter press where the insoluble materials (referred to as residue in Table 1 below) are separated from the aqueous phase. The temperature is maintained at 60° C. for filtration. The solution exiting the filter press is transferred to a neutralisation reactor. Calcium carbonate is added at a Ca / P molar ratio of 1 and a pH of 2.5-3.0. After 45 minutes of reaction and formation of dibasic calcium phosphate (DCP), the neutralised reaction medium is then directed to a band filter. The resulting cake is dried and con...
example 3
[0161]Example 1 is repeated using Syrian ore containing a P205 content of 30.9%. Dibasic calcium phosphate having a P205 content of 42% by weight and calcium content of 27% by weight is obtained. The dibasic calcium phosphate obtained in Example 3 has the same characteristics, in terms of levels of radioactive elements, as the dibasic calcium phosphate of Example 1. Table 2 below details the metallic content in the dibasic calcium phosphate obtained from Example 3. The table also details the metallic contents of a dibasic calcium phosphate described in WO2004 / 002888 produced by a comparative method wherein the steps involving the digestion and filtration of the attack liquor are performed at room temperature.
TABLE 2Metallic content (in ppm) in the dibasic calciumphosphate of Example 3 and in the dibasic calciumphosphate of W02004 / 002888DCP (Ex. 3 -DCPInvention)(comparativeAs1.52.01Ba3.5n.d.Cd0.0960.65Co0.94n.d.Cr6673Cu3.81.3Hgn.d.Mg2023.5Mn3n.d.Mo0.83n.d.Ni2.23.5Pb1.33.7Sr240n.d.Tln...
example 4
Preparation of Single Superphosphate (SSP)
[0163]The dibasic calcium phosphate prepared in Example 1 can be used for the preparation of single superphosphate. The dibasic calcium phosphate was mixed with a sulphuric acid solution (0.5 molar equivalent relative to the quantity of dibasic calcium phosphate) in a double propeller mixer (operation in opposite direction). The single superphosphate (Ca(H2P04)2) obtained with a P205 content between 32 and 37% by weight is dried and sieved. The single superphosphate can be used as such as a fertilizer in a composition intended for agricultural use. The quantity of sulphuric acid may be adapted to form, in the presence of a bulking agent, single superphosphate having a P205 content between 16 to 18%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com