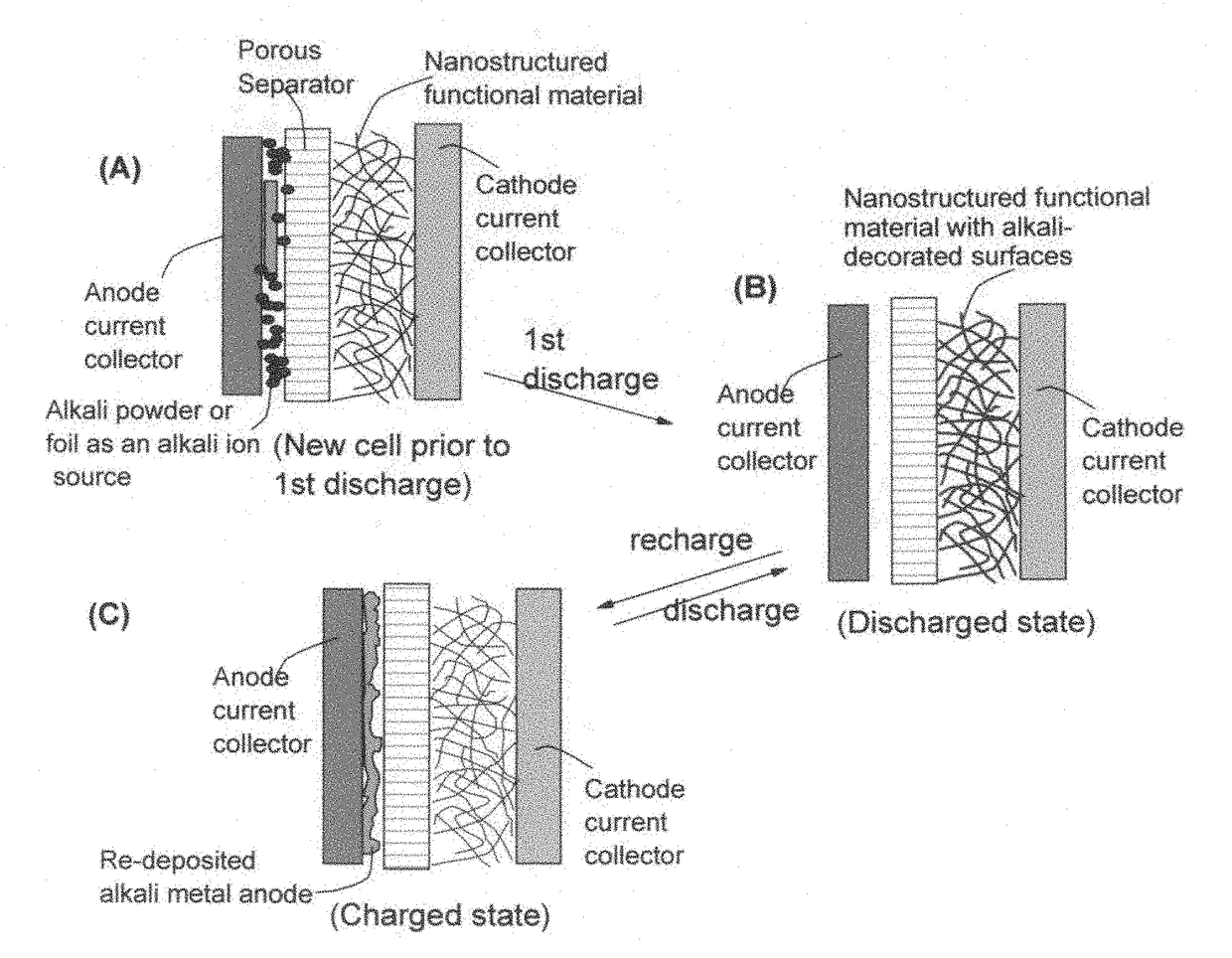
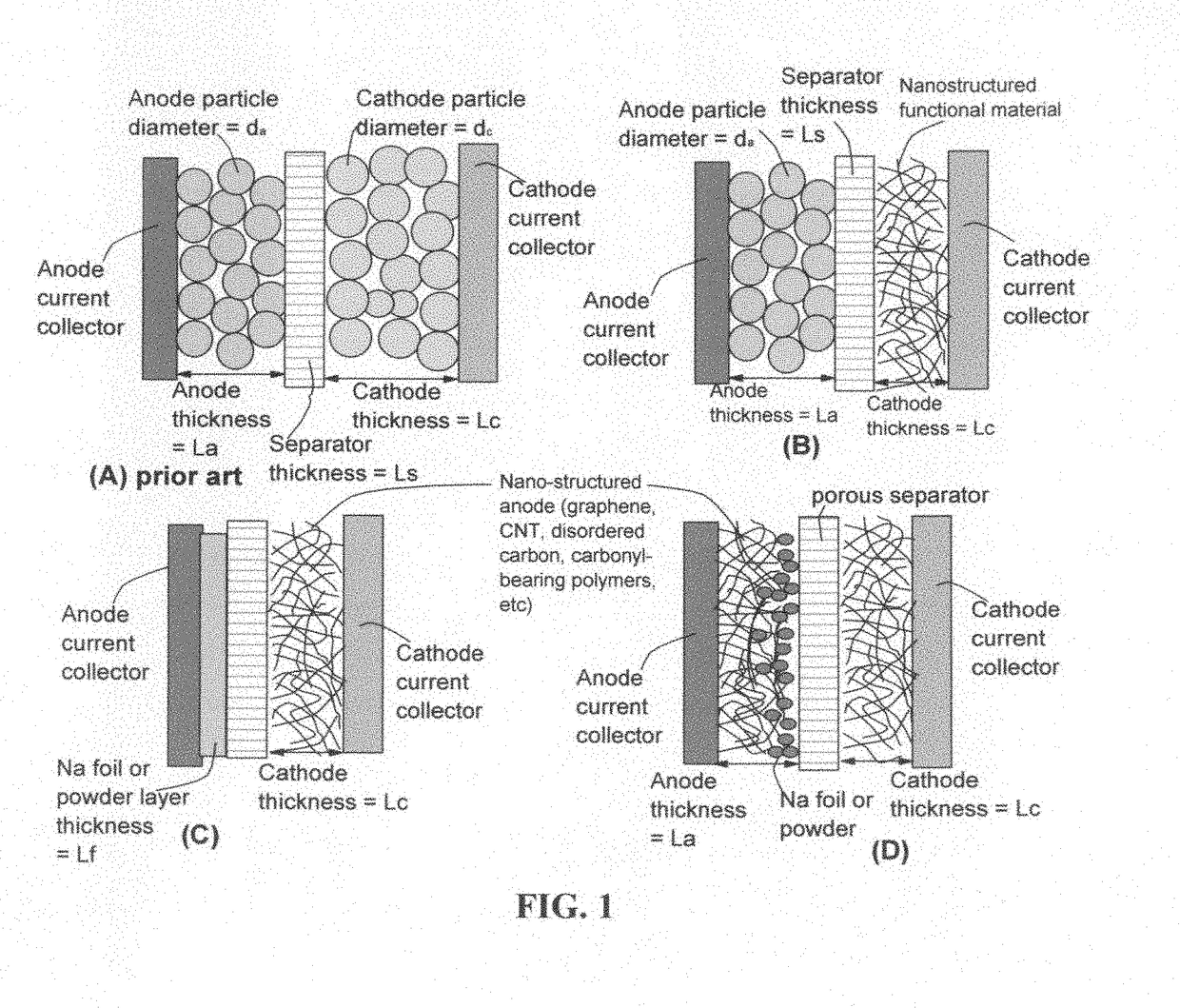
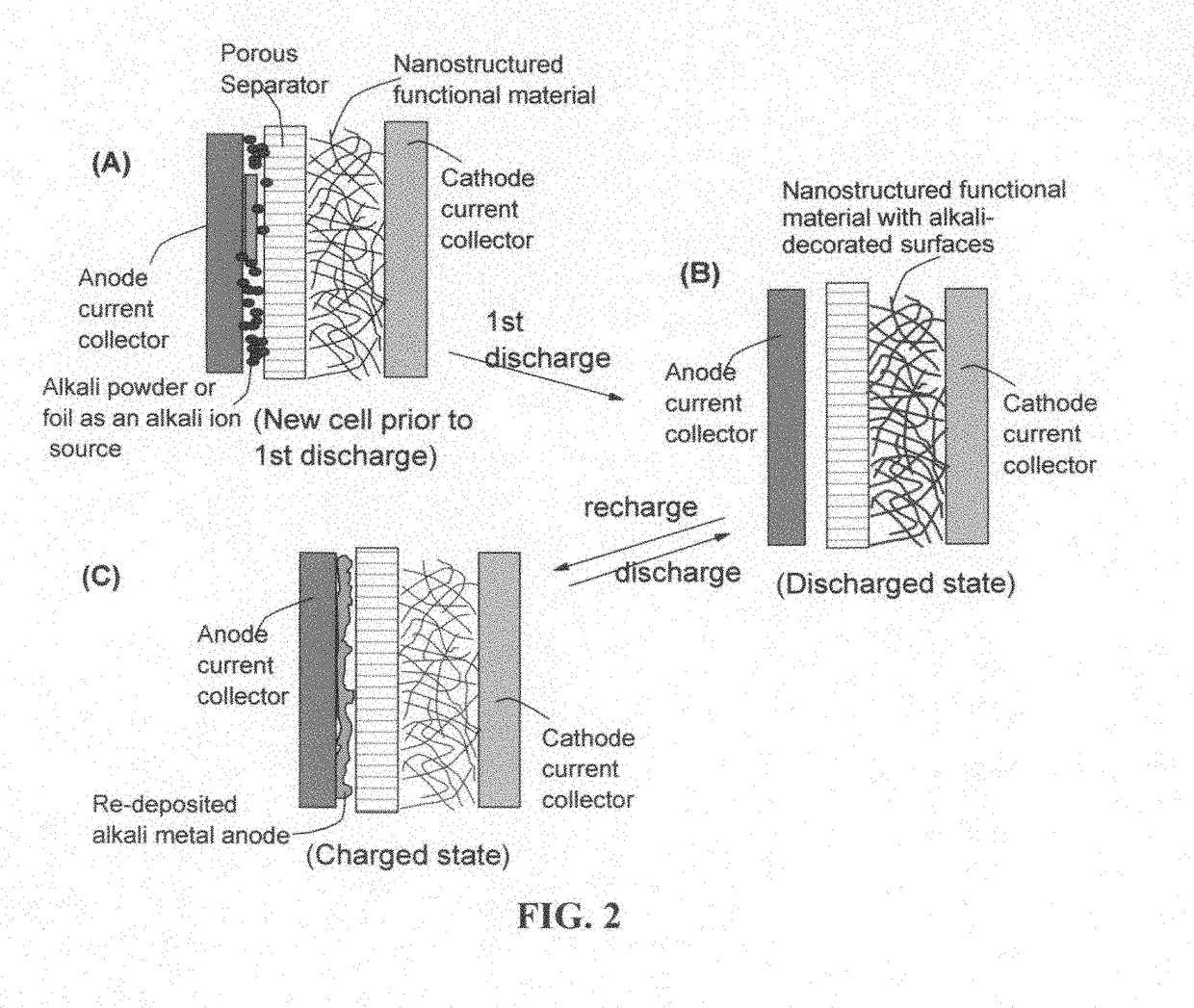
Partially and fully surface-enabled alkali metal ion-exchanging energy storage devices
a metal ion-exchanging battery and partially surface-enabled technology, applied in the direction of positive electrodes, electrochemical generators, cell components, etc., can solve the problems of reducing the power density of conventional supercapacitors (symmetric and asymmetric) and limiting the chemical reaction, so as to eliminate the potential formation of dendrites and uniform electric fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
NGPs from Sulfuric Acid Intercalation and Exfoliation of MCMBs
[0170]MCMB 2528 microbeads (Osaka Gas Chemical Company, Japan) have a density of about 2.24 g / cm3; a median size of about 22.5 microns, and an inter-planar distance of about 0.336 nm. MCMB 2528 (10 grams) were intercalated with an acid solution (sulfuric acid, nitric acid, and potassium permanganate at a ratio of 4:1:0.05) for 24 hours. Upon completion of the reaction, the mixture was poured into deionized water and filtered. The intercalated MCMBs were repeatedly washed in a 5% solution of HCl to remove most of the sulphate ions. The sample was then washed repeatedly with deionized water until the pH of the filtrate was neutral. The slurry was dried and stored in a vacuum oven at 60° C. for 24 hours. The dried powder sample was placed in a quartz tube and inserted into a horizontal tube furnace pre-set at a desired temperature, 600° C. for 30 seconds to obtain exfoliated graphite. The exfoliated MCMB sample was subjected...
example 2
Preparation of Nano-Structured, Functionalized Soft Carbon (One Type of Disordered Carbon)
[0172]Functionalized soft carbon was prepared from a liquid crystalline aromatic resin. The resin was ground with a mortar, and calcined at 900° C. for 2 h in a N2 atmosphere to prepare the graphitizable carbon or soft carbon. The resulting soft carbon was mixed with small tablets of KOH (four-fold weight) in an alumina melting pot. Subsequently, the soft carbon containing KOH was heated at 750° C. for 2 h in N2. Upon cooling, the alkali-rich residual carbon was washed with hot water until the outlet water reached a pH value of 5-7. The activated soft carbon (porous and nano-structured) was then immersed in a 90% H2O2-10% H2O solution at 45° C. for an oxidation treatment that lasted for 2 hours. Then, the resulting partially oxidized soft carbon was immersed in HCOOH at room temperature for functionalization for 24 hours. The resulting porous, functionalized soft carbon was dried by heating at ...
example 3
Nano-Structured Soft Carbon-Based Surface-Enabled Alkali Battery Devices
[0173]Fully surface-enabled coin cells using functionalized soft carbon as a cathode and functionalized soft carbon as a nano-structured anode (plus a small piece of potassium foil as a potassium source implemented between a current collector and a separator layer, Sample-1A) were made and tested. The separator was one sheet of micro-porous membrane (Celgard 2500). The current collector for each of the two electrodes was a piece of carbon-coated aluminum foil. The nano-structured electrode was a porous composite composed of 85 wt. % functionalized soft carbon (+5% Super-P and 10% PTFE binder coated on Al foil). The electrolyte solution was 1 M KPF6 dissolved in a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) with a 3:7 volume ratio. The separator was wetted by a minimal amount of electrolyte to reduce the background current. Cyclic voltammetry and galvanostatic charge-discharge measurements of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com