Cyclized cytokine and method for producing same
a cytokine and cyclization technology, applied in the direction of peptide/protein ingredients, drug compositions, extracellular fluid disorder, etc., can solve the problems of inconvenient patient care, inconvenient medication compliance, and undesirable metabolic half-life of bioactive proteins, and achieve high cyclization efficiency, increase thermostability and protease resistance, and small number of amino acids added
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
To Design Improved G-CSFs
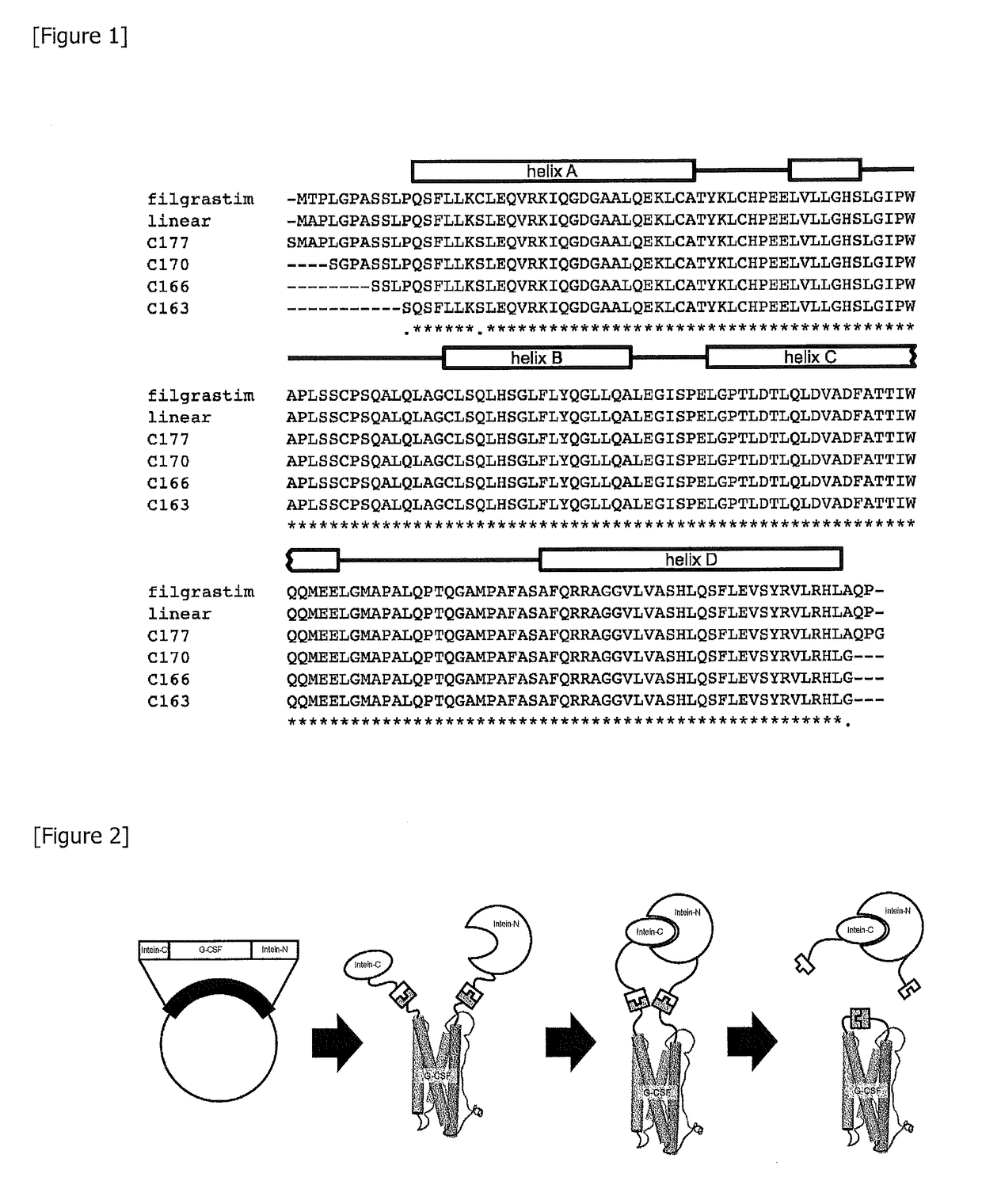
[0148](1) As used herein, a linear control G-CSF, which is a starting material for improvement, is defined as follows. Specifically, the linear control G-CSF is a linear polypeptide consisting of 175 amino acids set forth in SEQ ID NO: 1. When the amino acid sequence of the linear control G-CSF is compared with the amino acid sequence (SEQ ID NO: 14) of filgrastim, threonine at position 2 and cysteine at position 18 are replaced by alanine and serine, respectively. A previous report has demonstrated that these mutations do not affect the activity of G-CSF (Reference Document 3).
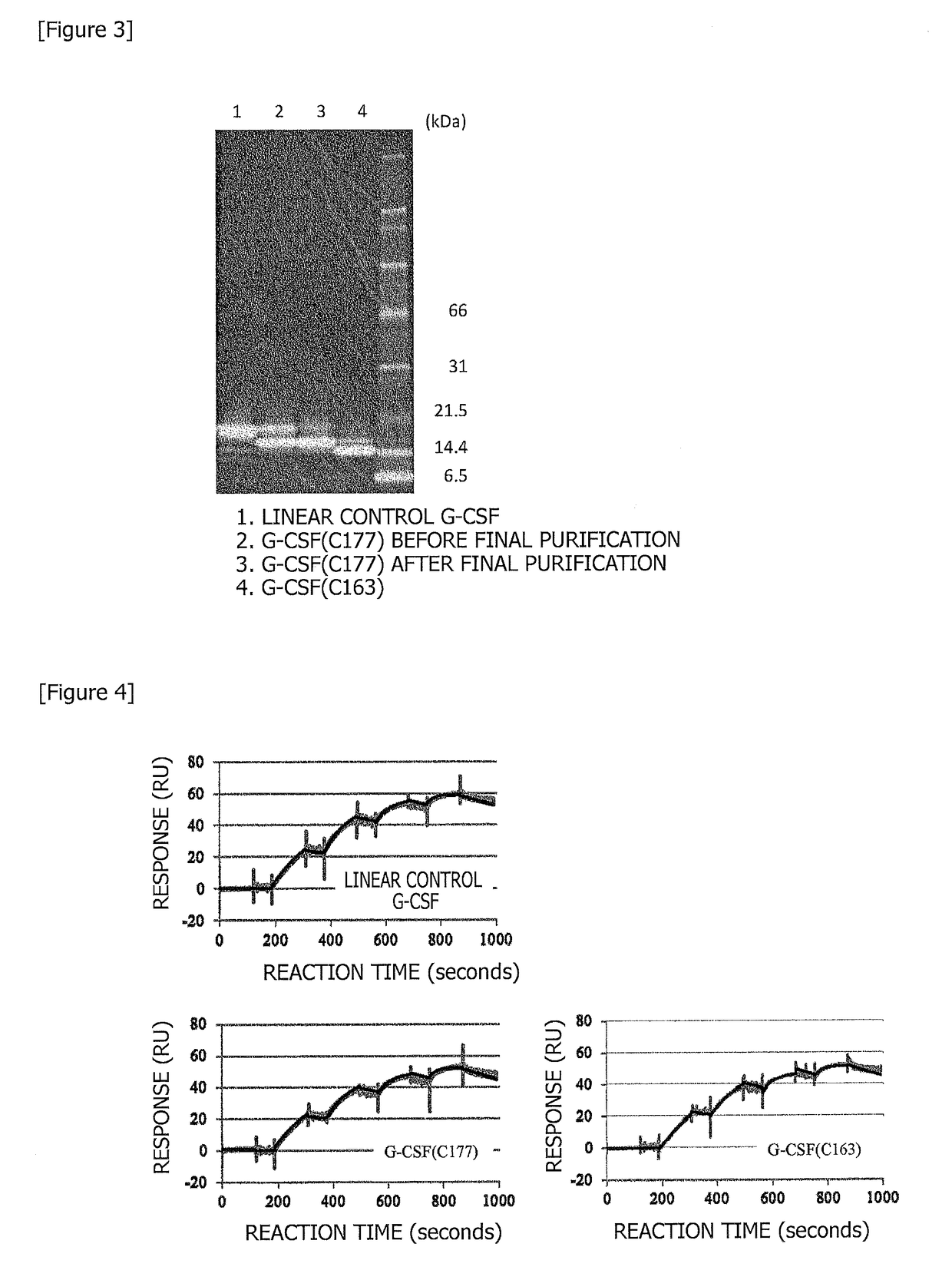
[0149](2) In order to make a polypeptide of interest cyclic by using the catalytic function of inteins, a residue of at least one of the N-terminus and the C-terminus should be serine or cysteine. In addition, if the residue at the C-terminus is proline, it is known that the reaction fails to proceed. Here, as a mutant modified so as to make the inteins reaction proceed by introducing...
example 2
To Synthesize Linear Control G-CSF-Expression Plasmid
[0154](1) A chemically synthesized nucleic acid sequence (SEQ ID NO: 25) encoding the amino acid sequence (SEQ ID NO: 1) of the linear control G-CSF was purchased.
[0155](2) As the chemically synthesized nucleic acid as a template, PCR amplification was carried out using two primers (SEQ ID NOs: 26 and 27). The resulting PCR product was purified and digested by restriction enzymes NcoI and BamHI. Next, an E. coli vector pET16b was digested by restriction enzymes NcoI and BamHI and a band at or near 5600 bp was excised and purified. The band was dephosphorylated using_E. coli alkaline phosphatase. The two samples purified were ligated using T7 DNA ligase.
[0156](3) E. coli DH5a was transformed with the resulting ligation product of (2) and was cultured on an LB plate medium containing 100 μg / m1 of ampicillin. The resulting transformants were subjected to colony PCR and DNA sequencing (GE Healthcare Bioscience, BigDye Terminator v3.1)...
example 3
To Construct Inteins Vector
[0157](1) A synthetic gene was purchased having a nucleotide sequence (SEQ ID NO: 28) encoding the inteins sequence (a sequence containing, in sequence, SEQ ID NO: 15 and SEQ ID NO: 16) derived from Nostoc punctiforme.
[0158](2) The synthetic gene of (1) was mixed with restriction enzymes NcoI and XhoI and was subjected to a cleavage reaction at 37° C. for 4 h. After gel electrophoresis, a band at or near 450 bp was excised and purified. Next, an E. coli vector pET16b was digested by NcoI and XhoI and a band at or near 5600 bp was excised and purified. The band was dephosphorylated using E. coli alkaline phosphatase. The two samples purified were ligated using T7 DNA ligase.
[0159](3) E. coli DH5a was transformed with the resulting ligation product and was cultured on an LB plate medium containing 100 μg / m1 of ampicillin. The resulting transformants were subjected to colony PCR and DNA sequencing (GE Healthcare Bioscience, BigDye Terminator v3.1) and were t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com