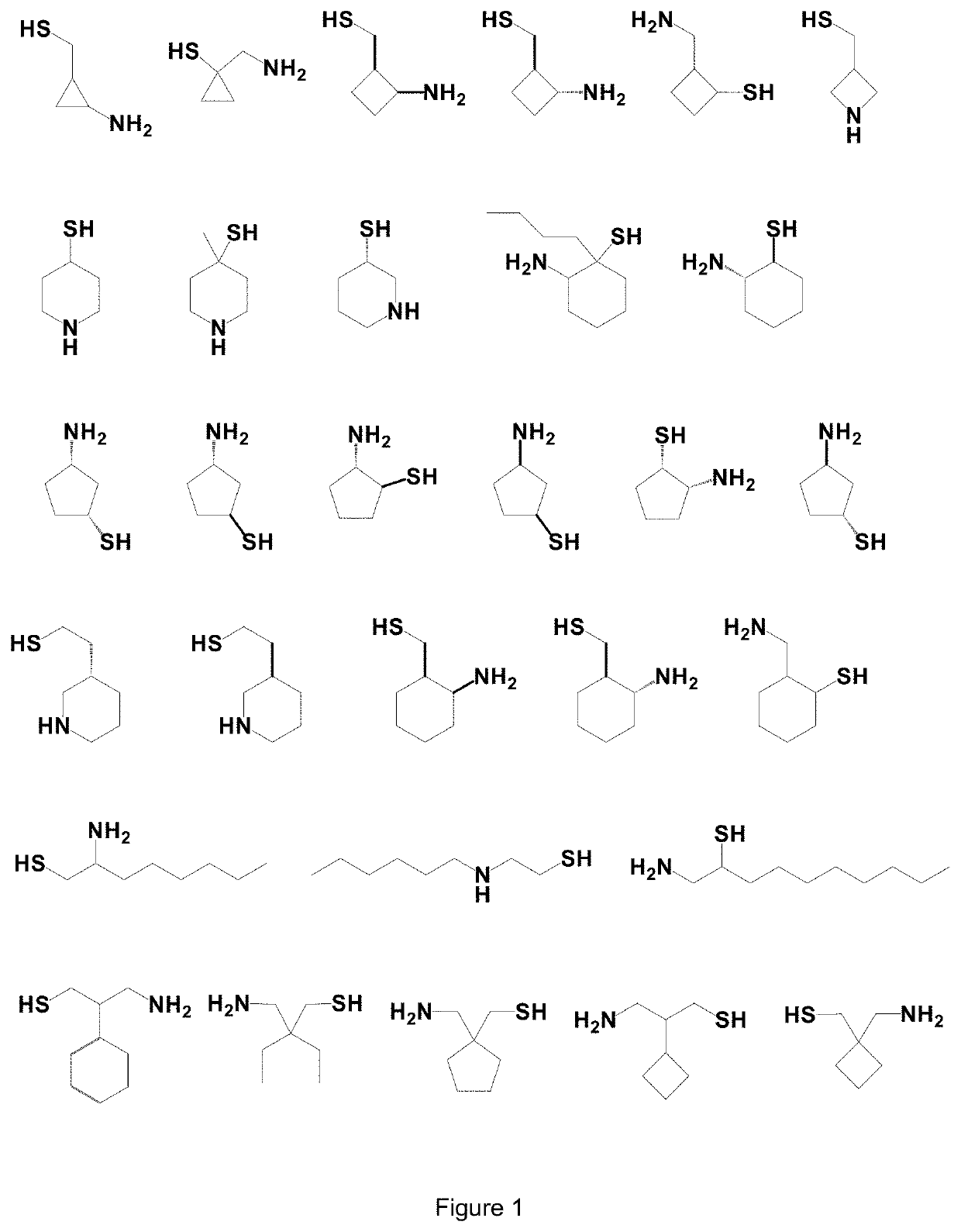
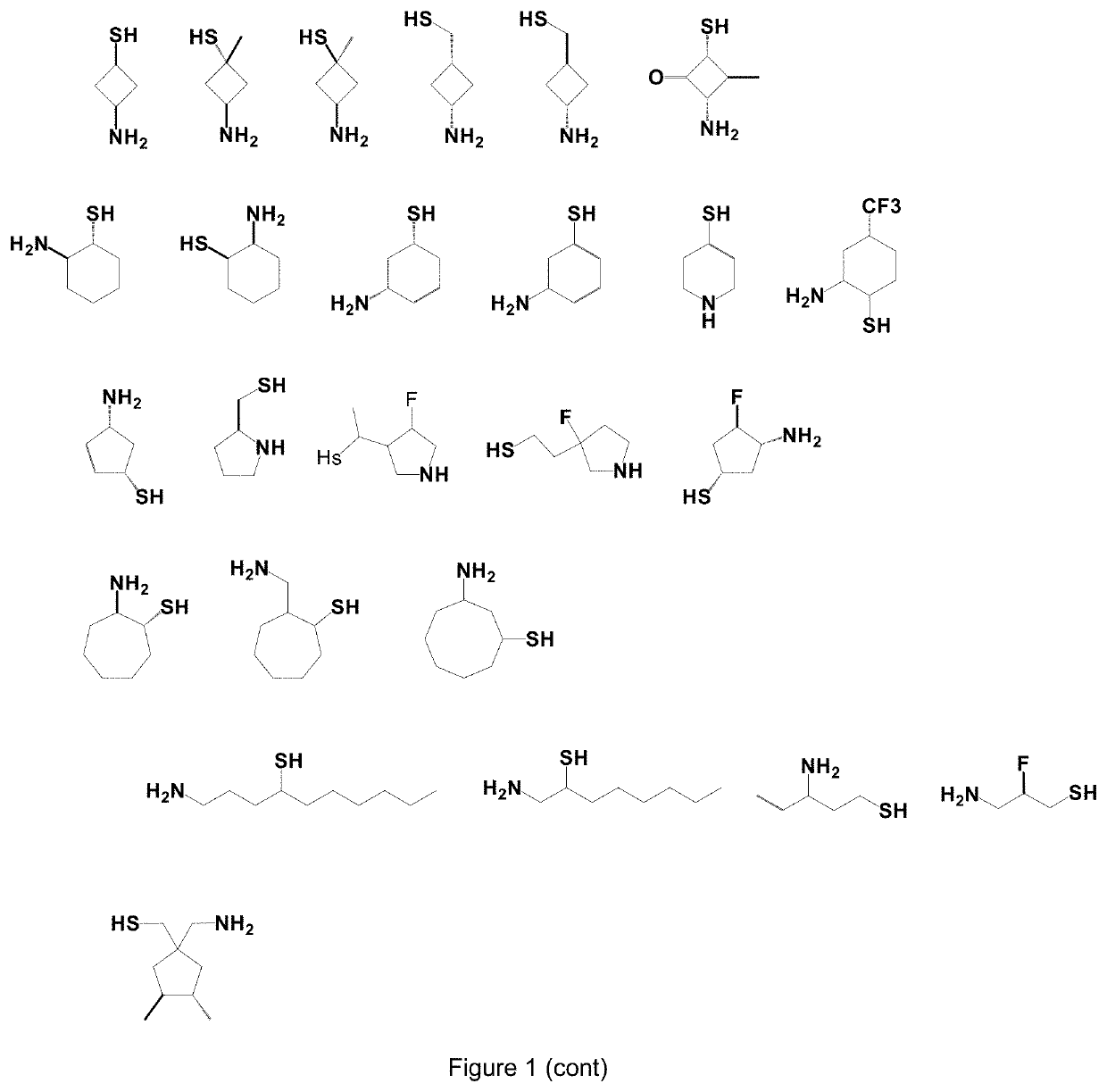
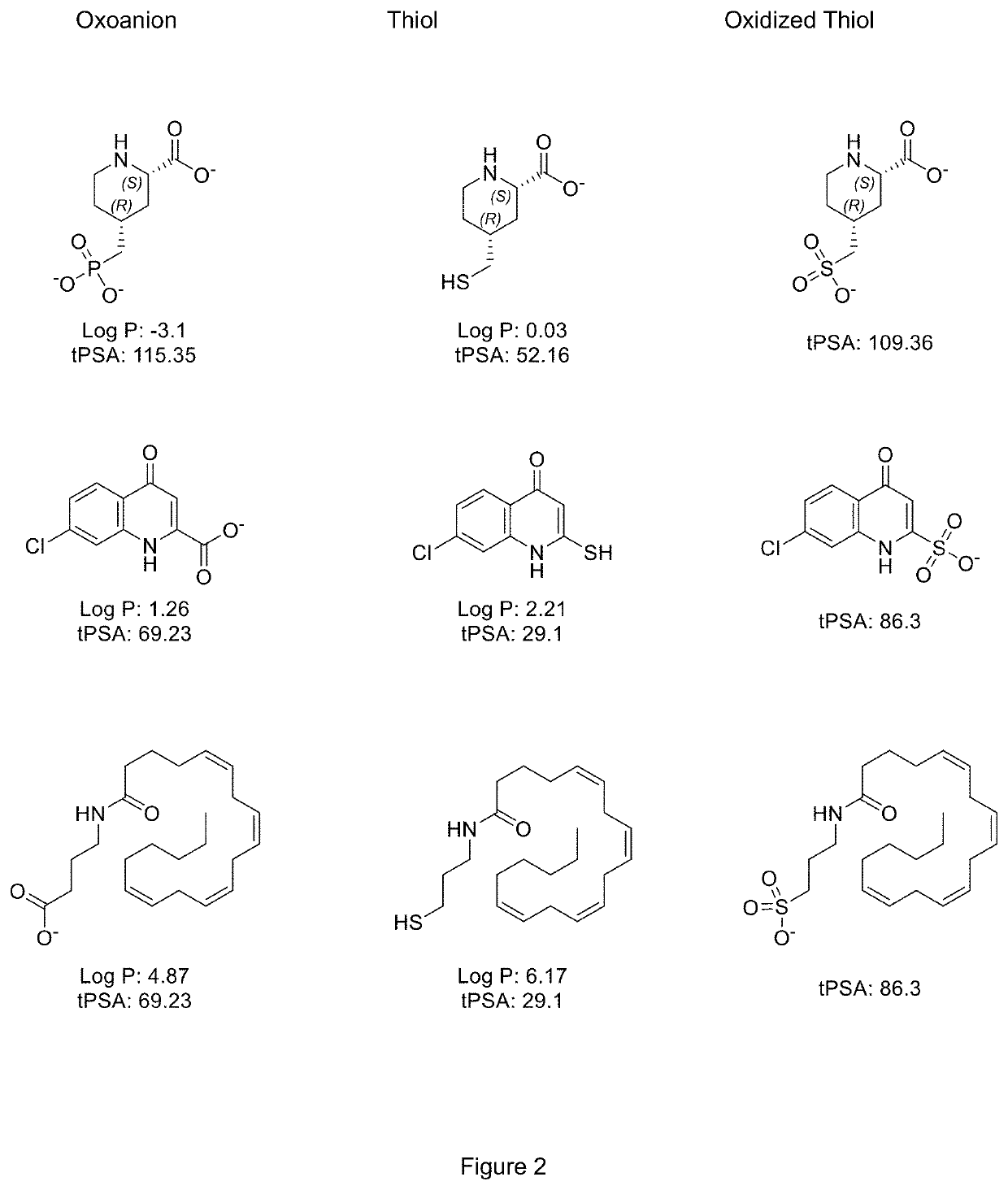
Use of Thiol Compounds to Treat Neurological Disease
a technology of neurological disease and thiol compounds, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, nervous disorders, etc., can solve the problems of danger when not properly controlled, and achieve the effects of reducing calcium transport, improving cell viability, and relieving mitochondrial stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Oxidative Stress and its Prevention by Captons
[0204]While the assay with Q111 striatal neurons may measure the ability to rescue sulfur starvation, the previous assay does not measure rescue from excitotoxicity and the effects on neurotransmission and neuroprotection mediated by the small thiol compounds. The ability of test compounds to inhibit glutamate-induced excitotoxicity (neuroprotection) in St-HdhQ111 / 111 cells is determined by incubating the small thiol compounds with cells for 60 min at 33° C., 95% (v:v) air / 5% (v:v) CO2 in BrainPhys complete media (STEMCELL Technologies, Vancouver, British Columbia, Canada). Following this, excitotoxicity is induced by the addition of 0.5 mM L-glutamate for 24 hours. Cell viability is assessed by measuring ATP levels, e.g., using a luminescent-based CellTitre Glo assay (Promega). Compound neuroprotection (% cell survival) is expressed as a % of the effect recorded with 100 μM cysteamine (denoted as 100% cell survival).
[0205]It is expected...
example 2
ggregation and its Prevention by Captons
[0206]The ability of test compounds to diminish aggregate formation in cells treated with hydrogen peroxide is determined. Briefly, MyCell SOD1 (G93A) neurons (CDI, Madison, Wis.) are cultured in 95% (v:v) air / 5% (v:v) CO2 in BrainPhys complete media (STEMCELL Technologies). Excitotoxic stress is induced by the addition of 0.5 mM L-glutamate for 2 hours, with and without capton or controls. Cells are harvested, pelleted and snap frozen. Frozen whole-cell pellets are homogenized in lysis buffer (25 mM Tris, pH 7.8, supplemented with protease inhibitors) at 4° C. by brief sonication and cleared by centrifugation at 18,000×g. Soluble SOD1-containing cleared supernatants are snap frozen for later analysis. Insoluble SOD1 (aggregated) is extracted from the lysed cell pellet. Pellets are re-suspended in 1 mL washing buffer (50 mM Tris HCl pH 7.4, 100 mM NaCl, 10% glycerol (v:v), 1% Triton X-100 (v:v), 0.5% NP-40 (v:v) with protease inhibitors by vor...
example 3
of Glutamate-Induced Excitotoxic Stress and Modulation of GABA Pathways by Oxidized Captons (o-Captons)
[0208]MyCell SOD1 (G93A) neurons (CDI, Madison, Wis.) are plated at a density of 20,000 cells / well in 384-well format in BrainPhys medium (STEMCELL Technologies, Vancouver, British Columbia, Canada) with antioxidants, 1 μg / mL laminin and neuronal growth factor supplements. Pre-coated poly-D-lysine (PDL) plates are coated with matrigel as a matrix according to standard protocol. Half the medium is changed every 3 days for 3 weeks. Cells are treated with either glutamate receptor antagonists, GABA receptor agonists, GABA receptor antagonists, or VGCC blockers in the presence or absence of either captons or o-captons for 30 min. Cells are then dosed with glutamate at 500 μM. At various timepoints, cells are assayed for viability using CellTiter-Glo 2.0 or calcium content using a total intracellular calcium kit or ROS using appropriate, commercially-available test kits. Cells are on ot...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| aggregation | aaaaa | aaaaa |
| oxidative stress | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com