Adipose tissue derived stromal cells for the treatment of neurological disorders
a stromal cell and adipose tissue technology, applied in the direction of fused cells, skeletal/connective tissue cells, peptide/protein ingredients, etc., can solve the problems of brain cell death, brain damage irreversible, high risk and discomfort of bone marrow stromal cells, etc., and achieve the effect of reducing the functional defici
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Hematopoietic Commitment by Adipose Tissue-Derived Stromal Cells
[0133]A. Stromal cells are isolated from human adipose tissue according to the methods described in U.S. patent application Ser. No. 09 / 240,029, Filed Jan. 29, 1999, now U.S. Pat. No. 6,153,432 (the contents of which are incorporated by reference), and using the modifications to the growth medium as described above. Briefly, human preadipocytes were isolated from adipose tissue removed by liposuction surgery according to the procedures previously described by Rodbell and Hauner (Rodbell (1967) and (1974); Hauner, supra). Preadipocytes from the stromal-vascular fraction were resuspended in DMEM (high glucose) media containing 10% fetal bovine serum, 5% chick embryo extract, and antibiotics and plated at 25,000 cells / well in each of the wells of a 96 well plate (150 μl / well). The cells were then placed in a 37° C. 5% CO2 incubator and allowed to settle overnight. The cells are cultured as primary cultures for a period of ...
example 2
Astroglial Commitment by Human Adipose Tissue-Derived Stromal Cells
[0137]A. Stromal cells are isolated from human adipose tissue according to the methods described above. The cells are cultured as primary cultures for a period of up to 5 days following initial plating in a medium composed of, but not limited to, DMEM (high glucose) media containing 10% fetal bovine serum, 5% chick embryo extract, and antibiotics at 37° C. Cells are harvested by trypsin / EDTA digestion prior to differentiation / implantation.
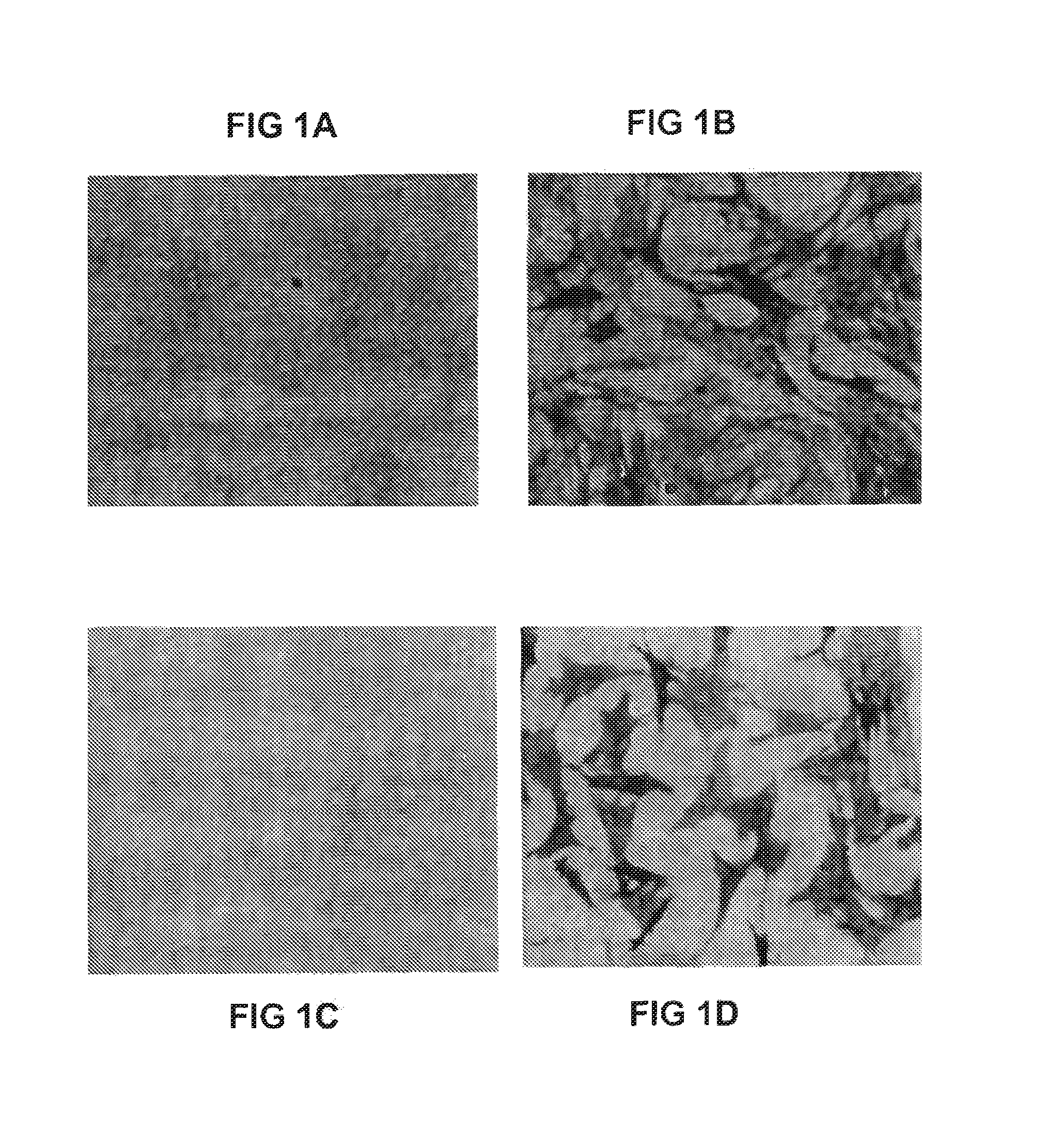
[0138]Cells are transplanted into the central nervous system of immunodeficient mice or rats. Nude / beige or SCID mice or nude rats are anesthetized in a sealed chamber using 3% halothane in oxygen; anesthesia is maintained by intramuscular injection of 6 mg / kg of xylozine and 60 mg / kg of ketamine. The animals are transferred to a sterotaxic apparatus in a clean field. A 2-to-5-mm incision is made in the scalp 2 mm lateral to the bregma. A burr hole is made in the bone 3 mm lateral t...
example 3
Neuronal Commitment by Human Adipose Tissue-Derived Stromal Cells
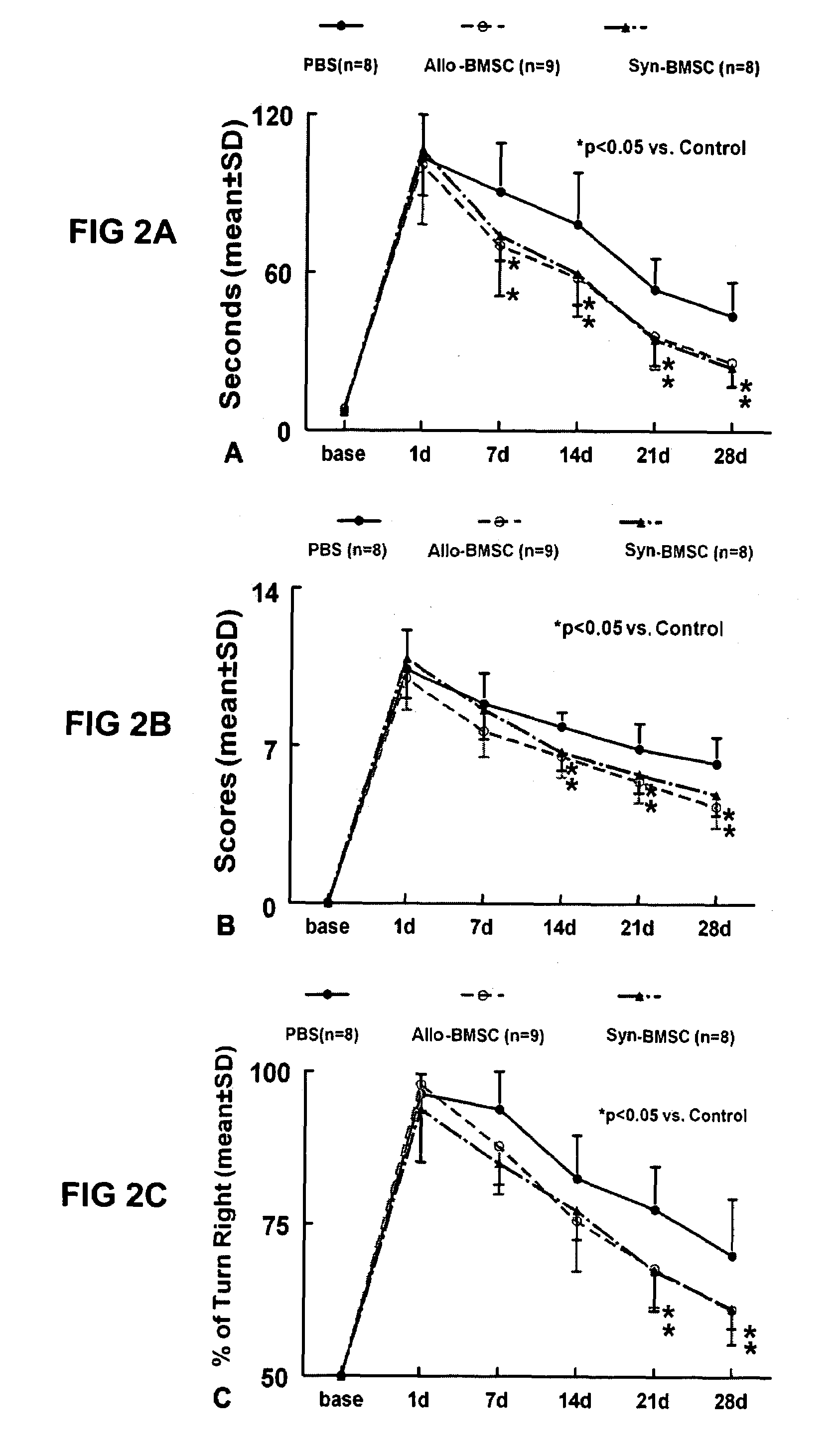
[0141]Improved Repair and Functional Recovery in a Traumatic Nervous System Injury
[0142]Stromal cells are isolated from human adipose tissue of an individual patient for autologous or allogeneic transplantation to a histocompatible recipient according to the methods described above. The cells are cultured as primary cultures for a period of up to 5 days following initial plating in a medium composed of, but not limited to, DMEM (high glucose) media with 1 mM glutamine but without pyruvate, containing 10% fetal bovine serum, 10% newborn calf serum, nucleoside stocks, 0.1 mM 2-mercaptoethanol, 1000 units / ml of leukemia inhibitory factor and antibiotics at 37° C.
[0143]Cells are harvested by trypsin / EDTA digestion prior to differentiation / implantation. Cells are then plated on tissue culture plastic substrate coated with 0.1% sterile gelatin solution. Cells are harvested during rapid growth stage by 0.25% trypsin and 1 mM ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com