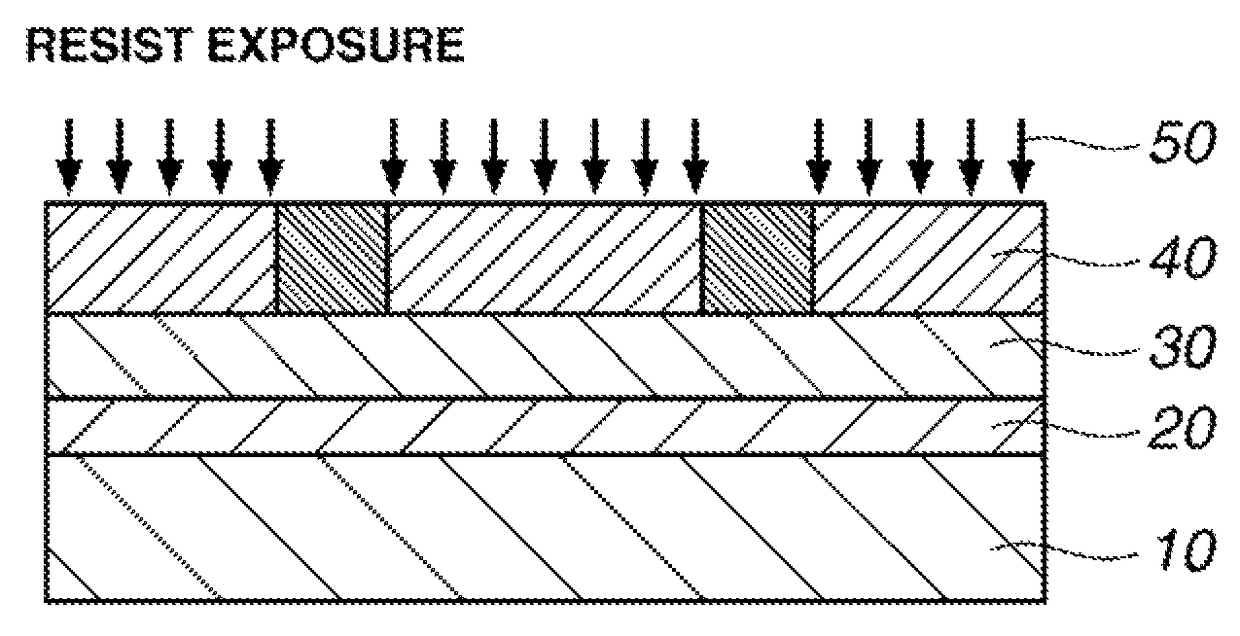
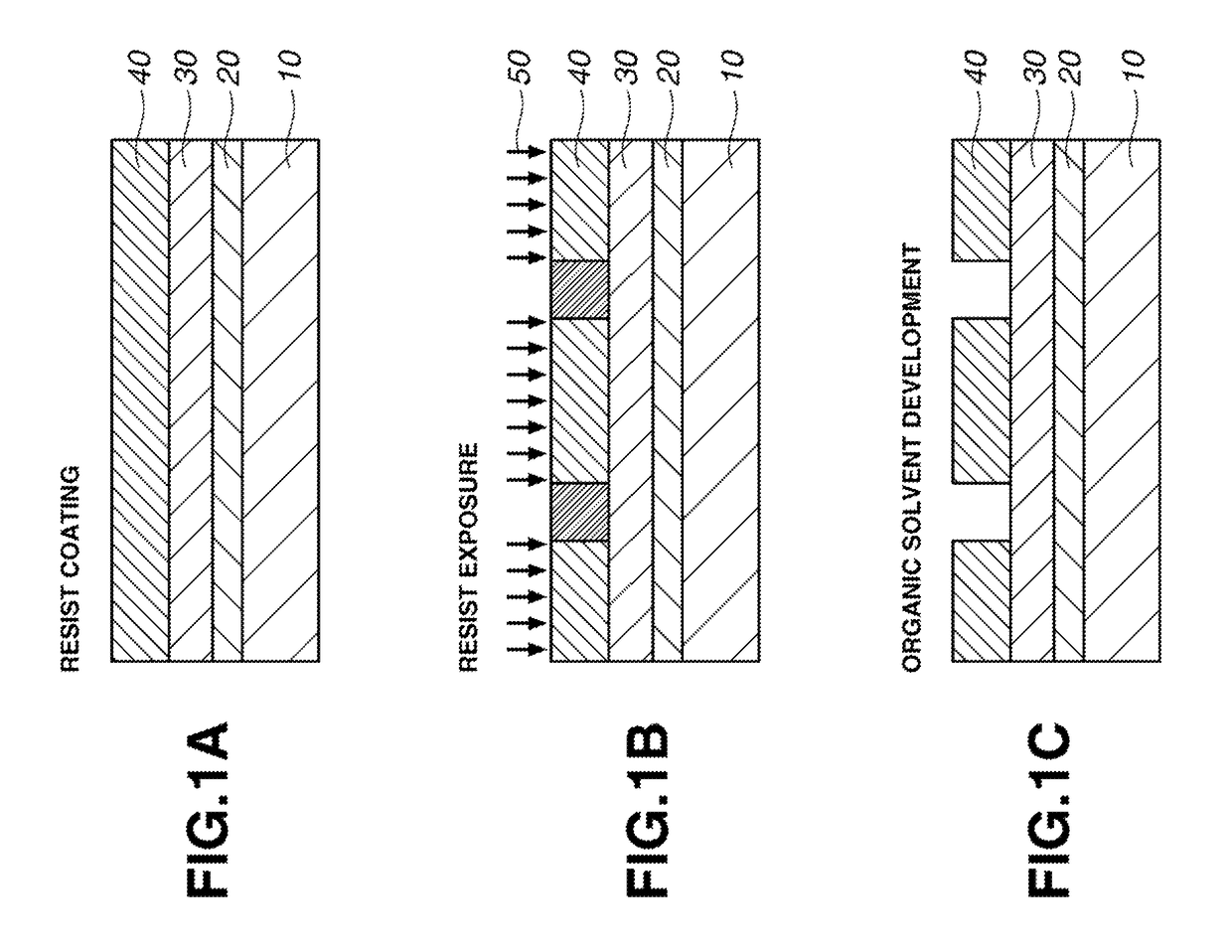
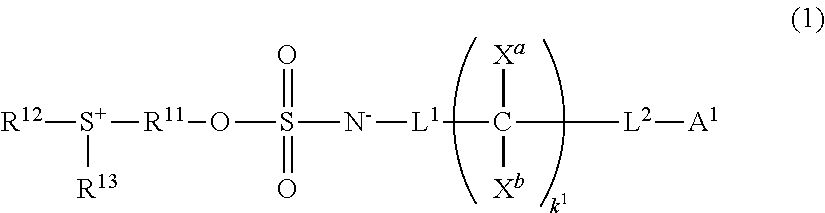Sulfonium salt, chemically amplified resist composition, and patterning process
a technology of resist composition and sulfonium salt, which is applied in the direction of photosensitive material processing, basic electric elements, electric apparatus, etc., can solve the problems of pfos, non-degradability, and several pfos, and achieves low pfos, improved mef, and low pfos. , the effect of improving the sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
[0270]Synthesis of PAG-1
[0271]PAG-1 was synthesized according to the following scheme.
[0272]
[0273]Under ice cooling, a solution of 2.98 g of trifluoromethanesulfonamide and 6.33 g of pyridine in 5 g of acetonitrile was added dropwise to a mixture of 2.70 g of sulfuryl chloride and 5 g of acetonitrile. The reaction solution was stirred at room temperature for 1 hour for aging. Under ice cooling, a solution of 9.01 g of diphenyl(p-hydroxyphenyl)sulfonium p-toluenesulfate and 0.49 g of N,N-dimethylaminopyridine in 10 g of acetonitrile was added dropwise to the reaction solution. The reaction solution was heated at 80-100° C. in an oil bath for 4 days for aging. The solution was allowed to cool down to 50° C. Thereafter, 4.88 g of meso-erythritol, 3.16 g of pyridine, and 5 g of acetonitrile were added to the solution, which was stirred at 80° C. for 3 hours. Water, 20 g, was added to the solution, acetonitrile was removed by vacuum concentration, and the concentrate was extracted with 1...
synthesis example 2
[0278]Synthesis of PAG-2
[0279]PAG-2 was synthesized according to the following scheme.
[0280]
synthesis example 2-1
[0281]Synthesis of Intermediate A
[0282]Under ice cooling, a solution of 2.98 g of trifluoromethanesuifonamide and 6.01 g of pyridine in 10 g of acetonitrile was added dropwise to a mixture of 2.70 g of sulfuryl chloride and 10 g of acetonitrile. The reaction solution was stirred under ice cooling for 5 minutes and at room temperature for 1 hour. Under ice cooling, a solution of 4.33 g of 1-naphthol and 0.12 g of N,N-dimethylaminopyridine in 20 g of acetonitrile was added dropwise to the reaction solution. The solution was stirred at 70° C. for 5 days for aging. Methanol, 5 g, was added to the reaction solution, which was aged at 70° C. for 24 hours. Acetonitrile was removed by vacuum concentration, and 80 g of MIBK was added to the concentrate, which was washed three times with 40 g of water. To the organic layer, 4.09 g of benzyltrimethylammonium chloride and 40 g of water were added, followed by stirring for 1 hour. The organic layer was washed once with 40 g of 10 wt % benzyltrim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com