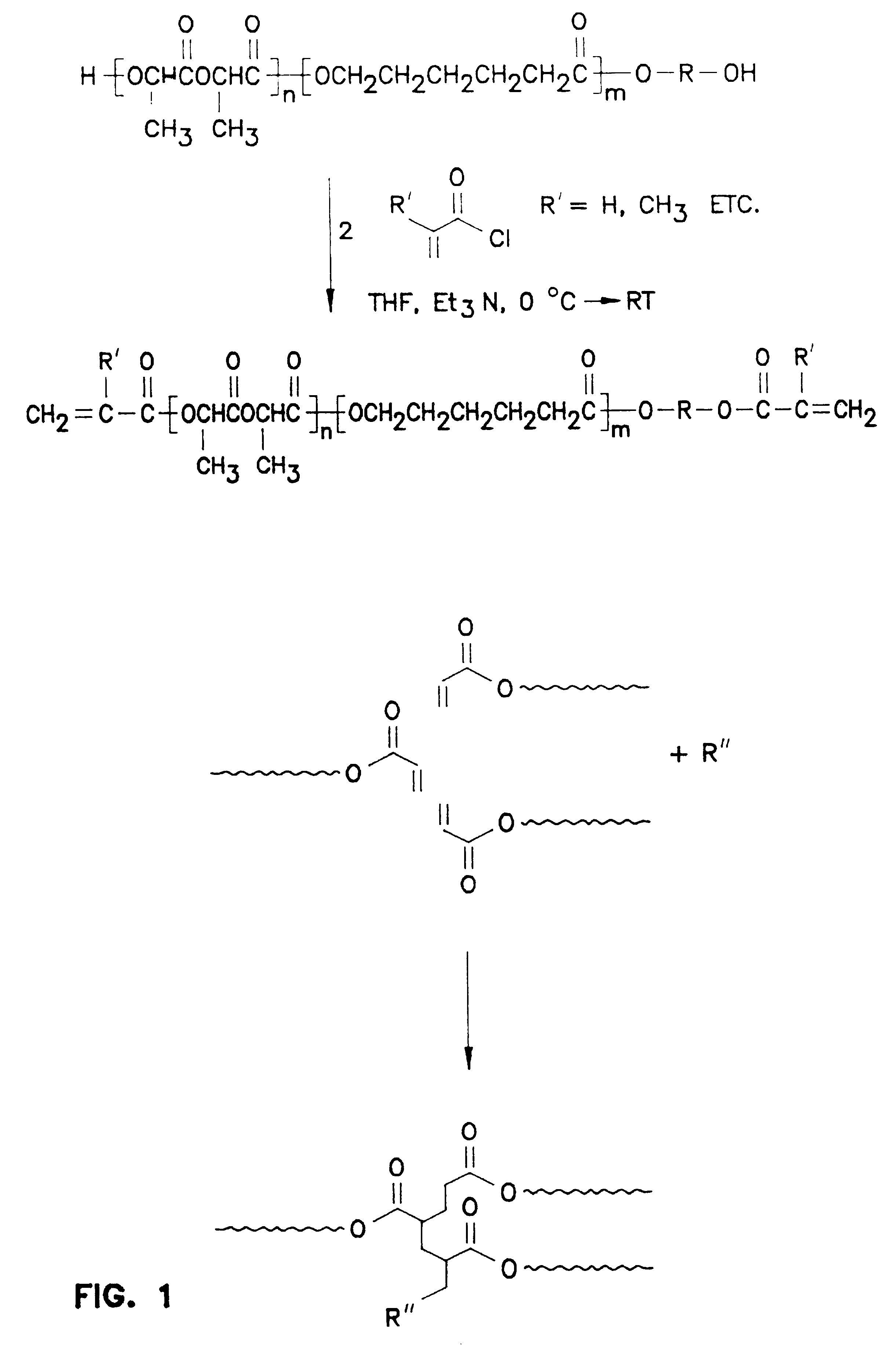
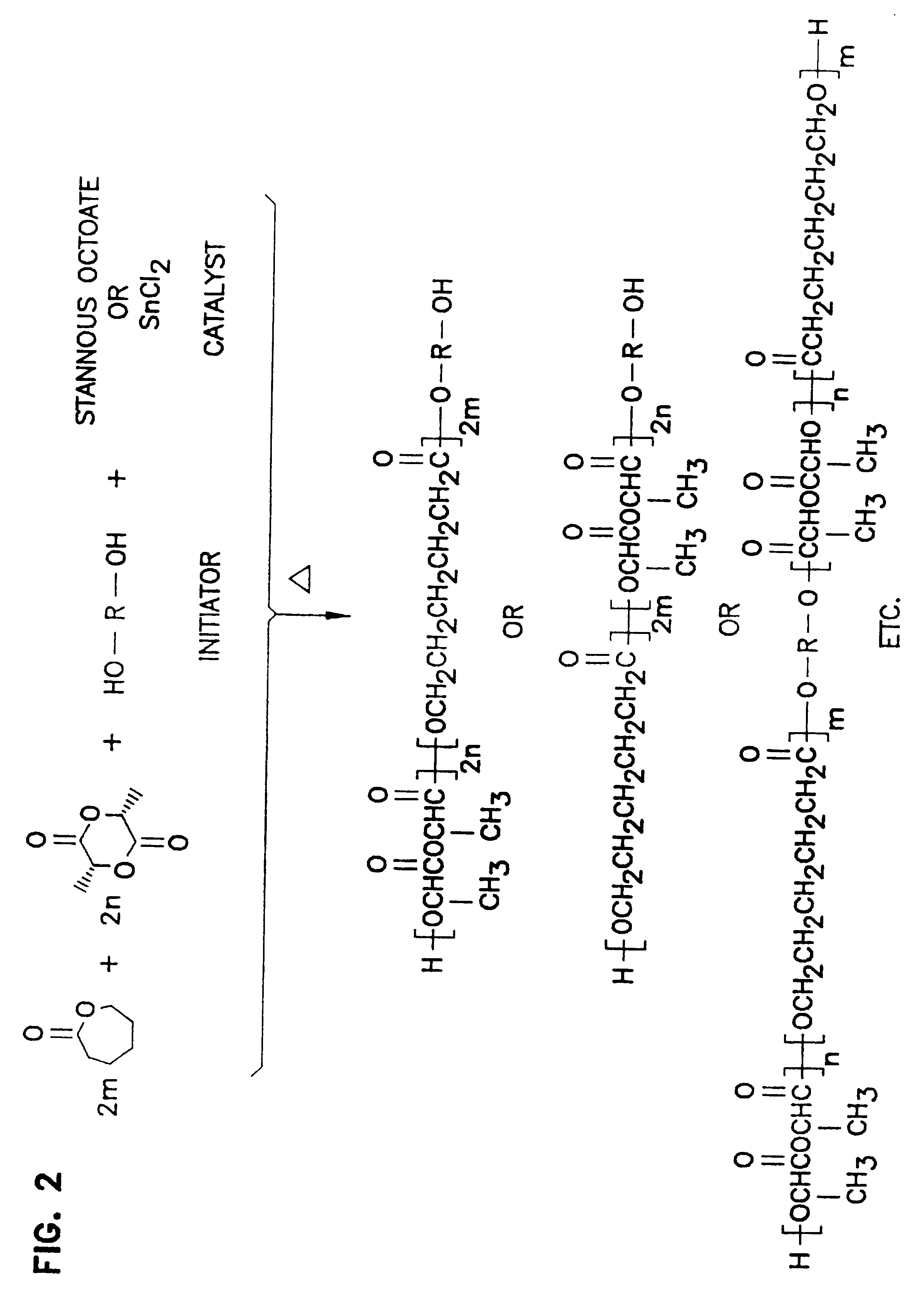
Biodegradable in-situ forming implants and methods of producing the same
a biodegradable, insitu forming technology, applied in the direction of prosthesis, surgical adhesives, dentistry, etc., can solve the problems of reluctance of patients to accept such implants or drug-delivery systems, important limitations of their use, and inability to meet the demand for biodegradable implants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Poly(DL-lactic acid) was prepared by the simple poly-condensation of lactic acid. No catalysts were used, and the reaction times were varied to produce polymers with different theoretical molecular weights. These polymers were designated as DL-PLA oligomers. A quantity of the solid oligomer was dissolved in NMP to give a 68:32 ratio of polymer to solvent. Sanguinarine chloride(SaCl), a benzophenanthridine alkaloid with antimicrobial activity especially toward periodontal pathogens, was added to the polymer solution to give a 2% by weight dispersion of the drug in the total mixture. The dispersion of drug and polymer solution was then injected into a dialysis tube (diameter of 1.5 mm) with a sterile disposable syringe without a needle. Each end of the 6-in. length of dialysis tubing was tied with a knot to prevent loss of the drug / polymer mass, and the tube with the injected material was placed in a pH 7 Sorenson's buffer receiving fluid maintained at 37.degree. C. Upon immersion in ...
example 2
Ethoxydihydrosanguinarine(SaEt), the ethanol ester of sanguinarine, was added to the same DL-PLA oligomer / NMP solution described in Example 1. SaEt dissolved in the polymer solution to give a homogeneous solution of drug and polymer. Approximately 250 .mu.L of the solution was added to receiving fluid and the release of drug measured as described in Example 1. The release of SaEt was slower than that for SaCl as expected because of its lower water solubility. After the first day, approximately 45% was released, 52% after 2 days, 60% after 5 days, 70% after 9 days, and 80% after 14 days.
example 3
Poly(DL-lactide) with an inherent viscosity of 0.08 dL / g and a theoretical molecular weight of 2,000 was prepared by the ring-opening polymerization of DL-lactide using lauryl alcohol as the initiator and stannous chloride as the catalyst. This polymer was then dissolved in NMP to give a 40% by weight polymer solution. SaCl was dispersed in the solution of this polymer in NMP to give a 1.5% by weight dispersion of the drug in the solution and the release rate determined as described in Example 1. The release rate of the drug from this higher molecular-weight polymer was slower than from the DL-PLA oligomer. After the first day, approximately 32% was released, 40% after 2 days, 45% after 5 days, and 50% after 15 days.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com