Antifish lymphocystis virus capsid protein monoclonal antibody, and its preparing method
A technology of lymphocyst virus and monoclonal antibody, which is applied in the interdisciplinary field of biology, immunology and molecular virology, to achieve the effect of direct observation, detailed positioning and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
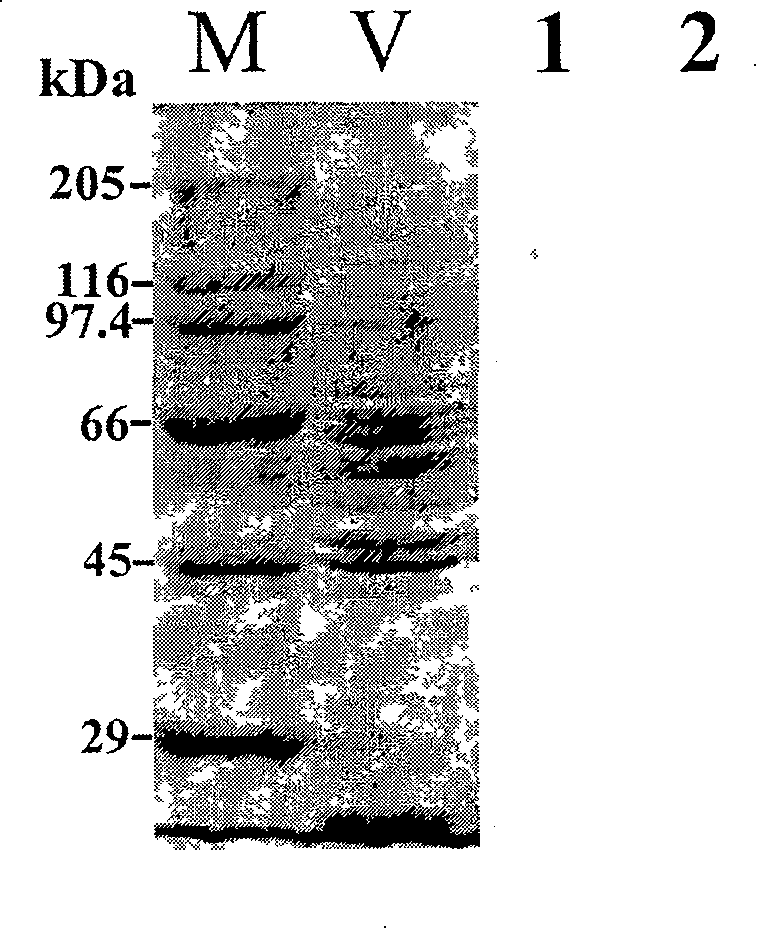
[0024] Development of VP monoclonal antibody against flounder LCDV:
[0025] 1.) LCDV virus purification:
[0026] (1) Take 10 g of cyst tissue of diseased fish, add appropriate amount of quartz sand and 10 times the volume of TNE buffer (0.05mol / L Tris-HCL; 0.15mol / L NaCl; 0.01mol / L EDTA; pH7.4), ice bath homogenate;
[0027] (2) The homogenate was centrifuged at 600g for 30min at 4°C, and the supernatant was taken;
[0028] (3) Centrifuge again at 1800g for 30min at 4°C; collect the supernatant;
[0029] (4) Centrifuge at 8000g for 2 hours at 4°C; discard the supernatant and collect the precipitate;
[0030] (5) Add an appropriate amount of TNE to the precipitate, place it lightly on the upper layer of a sucrose density gradient centrifuge tube consisting of 37%, 40%, 47%, 52%, 57%, and 62% (W / V), at 4°C, Centrifuge at 78000g for 2h;
[0031] (6) Carefully suck out the virus band with a disposable syringe, resuspend the virus band in TNE, and centrifuge at 78000g for 1 ...
Embodiment 2
[0054] Screening and identification by indirect immunofluorescence antibody method:
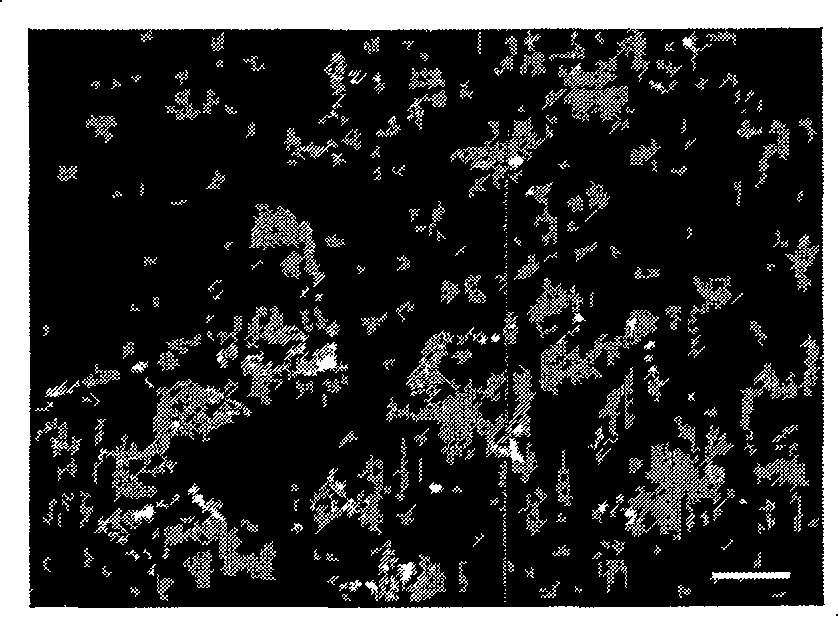
[0055] (1) Take a fresh cyst, cut it into small pieces of about 8 mm × 5 mm × 7 mm, wash with normal saline, absorb the water, embed it with tissue freezing embedding agent OCT, place it at -20°C for 30 min, and freeze it into sections with a thickness of 5 μm . Fix with acetone for 20 minutes, dry at room temperature, and freeze at -20°C for later use;
[0056] (2) Using the hybridoma cell culture supernatant as the first antibody, add it to the slice;
[0057] (3) Incubate in a wet box at 37°C for 45 minutes, soak in PBS-T (0.05% Tween-20) 3 times, 5 minutes each time;
[0058] (4) Goat anti-mouse IgG labeled with fluorescein isothiocyanate, added to the section;
[0059] (5) Incubate in a wet box at 37°C for 45 minutes, soak in PBS-T 3 times, 5 minutes each time;
[0060] (6) Cover the slides with glycerol, observe under a fluorescence microscope, and take pictures.
[0061] Results: ...
Embodiment 3
[0063] Colloidal gold-labeled immunoelectron microscope localization of the antigenic determinant specifically bound by the VP monoclonal antibody of the present invention
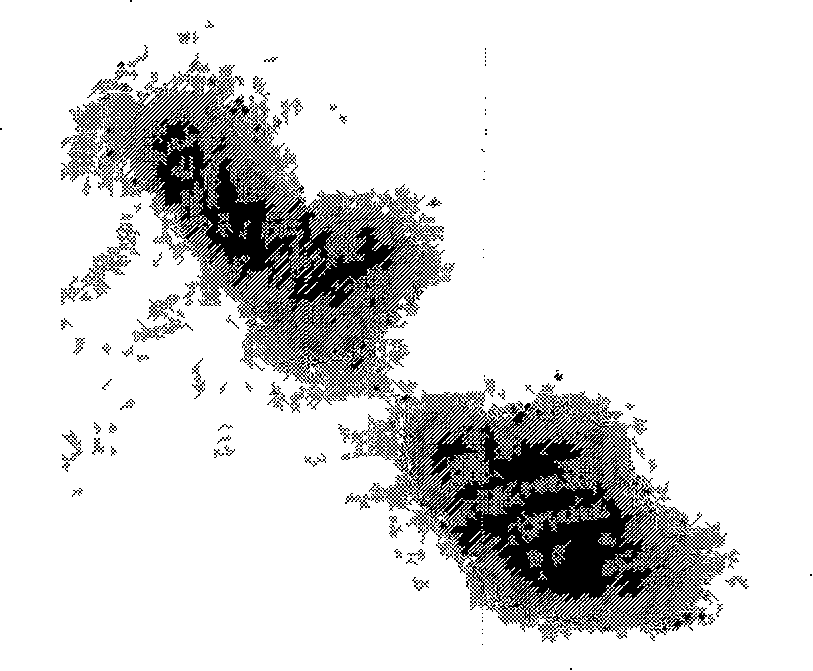
[0064] (1) Take 10 μl of purified virus suspension, drop it on a copper grid covered with Formvar membrane, let it rest for 5 minutes, absorb excess virus suspension, and soak in PBS-T;
[0065] (2) PBS containing 2% bovine serum albumin, block at 37°C for 1 hour, soak in PBS-T for 3 times,
[0066] (3) Add the VP116 monoclonal antibody of the present invention, incubate at 37°C for 45 minutes, and wash with PBS-T for 3 times;
[0067] (4) Add 15nm colloidal gold-labeled goat anti-mouse IgG, incubate at 37°C for 45min, soak in PBS-T for 3 times, and double distilled water for 3 times;
[0068] (5) Negative staining with 2% phosphotungstic acid for 1 min, absorb the remaining dye solution, and observe with electron microscope.
[0069] Results: Colloidal gold-labeled immunoelectron microscopy results show...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap