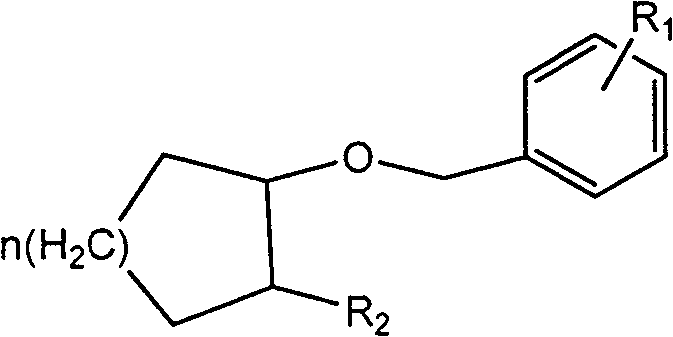
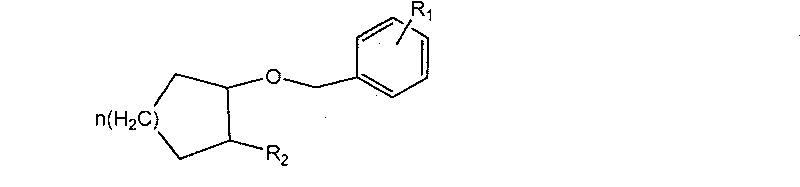
Preparation method of aliphatic cyclo benzylether
A technology of cyclobenzyl ether and aliphatic ring, which is applied in the field of synthesis of aliphatic cyclobenzyl ether, can solve problems such as post-processing troubles and yield improvement, and achieve the effects of shortened reaction time, convenient post-processing, and wide process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 450g (1.84mol) N-Boc protected cyclohexylamine alcohol and 429.5g (2.21mol, 1.2eq) benzyl bromide in the 5L four-necked round bottom flask that mechanical stirring, thermometer and spherical condenser are housed, 148.7g (0.368 mol, 0.2eq) trioctylmethylammonium chloride, and 1000ml of dichloromethane were stirred evenly; under stirring, 3.2kg of 50% sodium hydroxide solution was added in portions to the reactor, and the temperature of the mixture was controlled not to be higher than 40°C. After the 50% sodium hydroxide solution was added dropwise, the temperature was raised to 40°C and refluxed for 3-4 hours; after the reaction was completed, the temperature was lowered and the stirring was stopped. The layers were separated, retaining the dichloromethane layer. The dichloromethane layer was washed once with 300ml of water. After all the dichloromethane layers were distilled off under reduced pressure to remove most of the dichloromethane (minimum volume), n-hexane...
Embodiment 2
[0025] Add 200g of potassium hydroxide and 200g of water into a 2L four-necked round-bottomed flask equipped with mechanical stirring, a thermometer and a spherical condenser, stir to dissolve, cool to 40°C, then add 52.6g (0.354mol) azidocyclohexanol and 24.1 g (0.07mol, 0.2eq) tetrabutylammonium bisulfate, 500ml toluene and stir well. Under stirring, 49.2 g of benzyl chloride was added dropwise to the reaction mixture, and the temperature of the reaction mixture was controlled at 40±2°C. After the dropwise addition of benzyl chloride was completed, the temperature was raised to 60°C, heated and stirred for 4 hours, cooled down, the stirring was stopped, and layers were separated. The collected toluene solution was washed with 300 ml of semi-saturated saline, the chromatographic content of azidobenzyl cyclohexyl ether was 70%, and the chromatographic yield was 90%.
Embodiment 3
[0027] Add 120g of sodium hydroxide and 120g of water into a 1L four-necked round-bottomed flask equipped with mechanical stirring, a thermometer and a spherical condenser, stir to dissolve, cool down to 40°C, and then add 26.7g (0.21mol) of 2-azidocyclopentane Alcohol and 9.7g (0.042mol, 0.2eq) of trimethylbenzyl ammonium bromide, 35.2g (0.25mol, 1.2eq) of p-methylbenzyl chloride and 500ml of chlorobenzene were stirred, and the temperature of the reaction mixture was controlled at 70±2°C. After the reaction, the temperature was lowered, the stirring was stopped, and the layers were separated. The collected toluene solution was washed with 150 ml of semi-saturated saline, the chromatographic content of azidobenzyl cyclopentyl ether was 68%, and the chromatographic yield was 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com