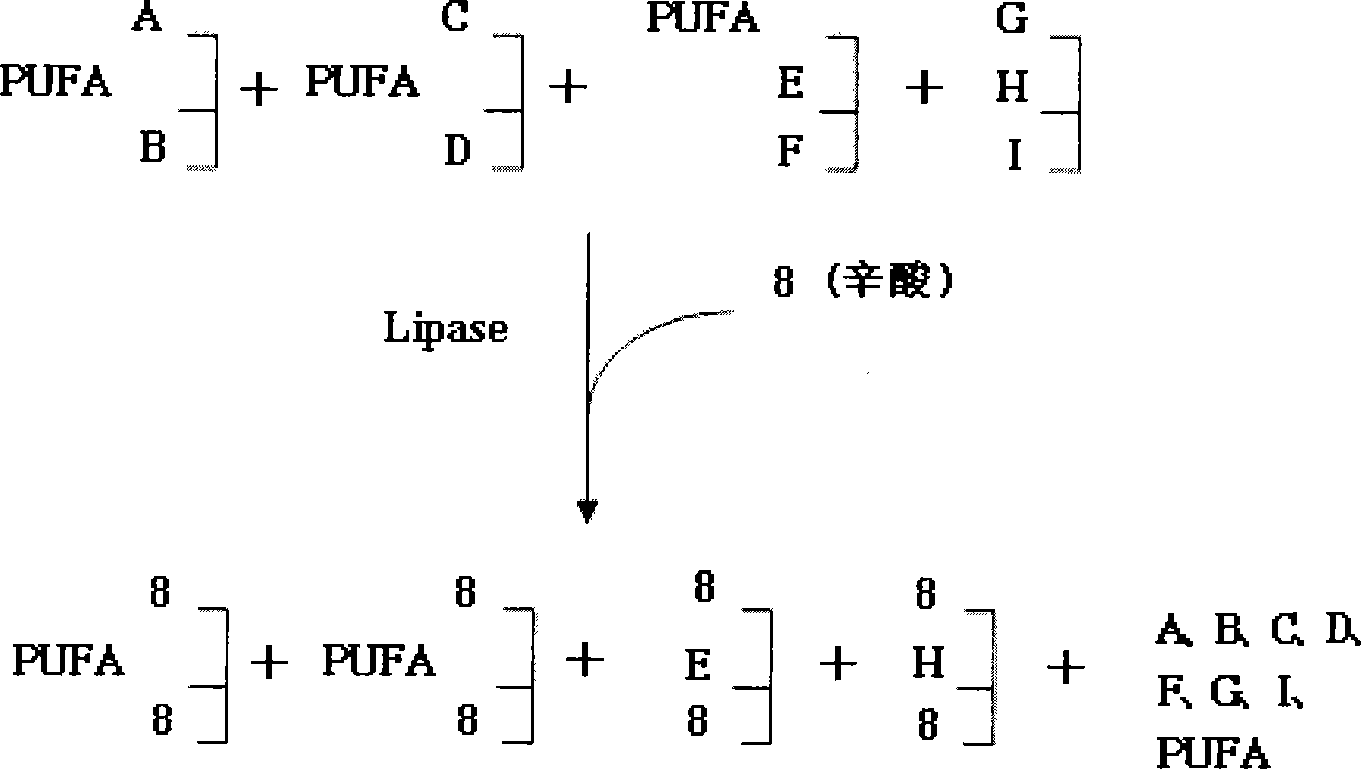
Process for producing triglyceride containing three residues of one highly unsaturated fatty acid and use thereof
A technology of unsaturated fatty acids and triglycerides, which is applied in the efficient and stable production of the above-mentioned triglycerides, triglycerides and their representative applications, which can solve the problem of high calories and achieve the effect of low calories and increased added value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1. Obtained by mutation treatment of Mortierella alpina (Mortierella alpina) SAM2268 A high-yield strain of triarachidonoylglycerol
[0080] Mortierella alpina ( Mortierella alpina ) SAM2268 (FERM P-17762) was inoculated into Czapek agar medium (0.2% NaNO 3 , 0.1% K 2 HPO 4 , 0.05% MgSO 4 ·7H 2 O, 0.05% KCl, 0.01% FeSO 4 ·7H 2 O, 3% sucrose, 2% agar, pH 6.0) in a 300mL large slant culture flask, cultured at 28°C for 2 weeks.
[0081] After the incubation, add 50 mL of sterilized water, shake and mix, filter with 4 layers of gauze, centrifuge at 8,000×g for 10 minutes, and suspend in 50 mM Tris buffer solution (pH 7.5) to prepare a spore suspension. in 1×10 6 0.5 mL of 0.5% NTG (N-methyl-N'-nitro-N-nitrosoguanidine) solution was added to 1.5 mL of spore suspension, and mutation treatment was performed at 28°C for 15 minutes. Add 10%Na 2 S 2 o 3 3 mL, centrifuged at 5,500×g for 10 minutes, washed with sterilized water to obtain NTG-treated spor...
Embodiment 2
[0084] Example 2. Comparison of production capacity of triarachidonoylglycerol
[0085] In 10 mL of liquid medium (glucose 2%, yeast extract 1%, palmitoleic acid 1% or nothing) contained in a 50 mL Erlenmeyer flask, inoculate an inoculation loop of the following 3 strains of Mortierella alpina (Mortierella alpina ), cultured at 28° C. with shaking at 120 rpm for 7 days.
[0086] (1) Mortierella alpine#1 (mutant strain obtained in Example 1)
[0087] (2)Mortierella alpina SAM 2268
[0088] (3)Mortierella alpina IFO 8568
[0089] After the cultivation, the bacterial cells were collected by filtration and dried. After drying a part of the grown bacterial cells, triglycerides were extracted with hexane according to a conventional method, and the hexane was distilled off to obtain triglycerides. The obtained triglyceride was subjected to high performance liquid chromatography, and the ratio of triarachidonoylglycerol (tri-AA) was determined by analyzing the molecular specie...
Embodiment 3
[0093] Example 3. Time-dependent changes in the production of triarachidonoylglycerol
[0094] Inoculate a loop of Mortierella alpina #1 into 10 mL of liquid medium (glucose 2%, yeast extract 1%, palmitoleic acid 1% or none) in a 50 mL Erlenmeyer flask, at 28°C Under 120rpm shaking culture for 6, 8, 10 days. After the cultivation, the bacterial cells were collected by filtration, and the ratio of triarachidonoylglycerol (tri-AA) and the ratio of arachidonic acid in total fatty acids were determined in the same manner as in Example 2. The results are shown in Table 2.
[0095] Table 2 Time-dependent changes of triarachidonoylglycerol (tri-AA) production in #1 strain
[0096] Training days (days)
[0097] Regarding the ratio of tri-AA, it was 30.1% on the 6th day, 45.9% on the 8th day, and 62.5% on the 10th day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com