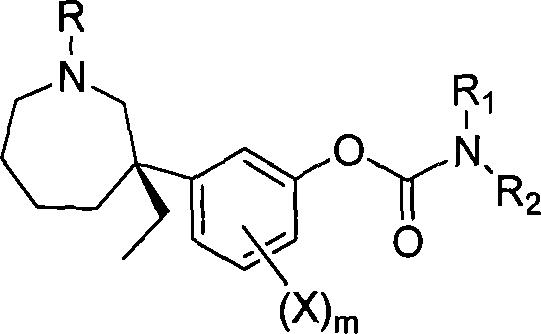
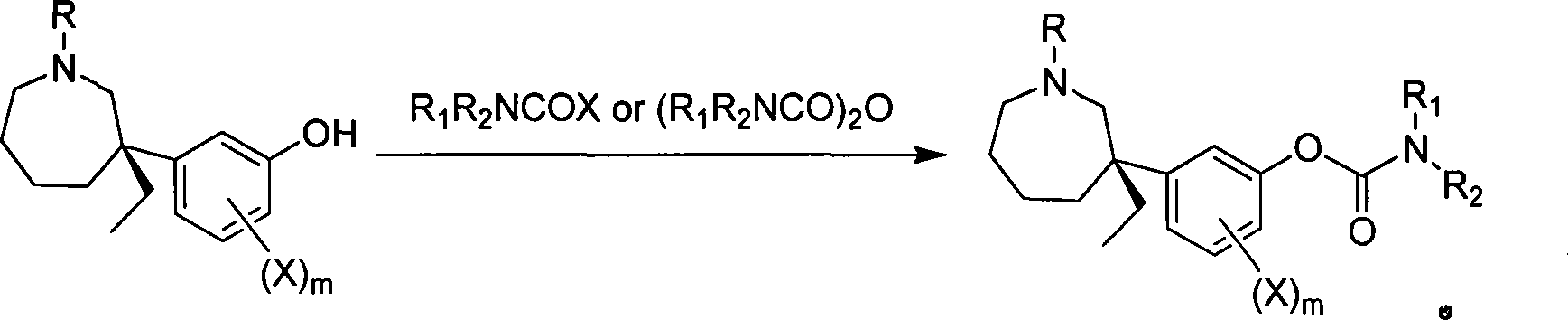(-)-meptazinol carbamate derivative and/or its salt and their prepn and use
A technology of carbamic acid and derivatives of mebutamol, which is applied in drug combinations, active ingredients of heterocyclic compounds, nervous system diseases, etc., to achieve the effects of good safety, low toxic and side effects, and high therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (-)-Maptafol is obtained by resolution, and its preparation method can be found in Chinese patent application (publication number: CN1850804). Embodiment 1 (-)-Mabutaol dimethyl carbamate (R 1 =CH 3 , R 2 =CH 3 ) preparation
[0037] Sodium hydride (80%, 0.15 g, 5.00 mmol) was added to acetonitrile, cooled, and a solution of (-)-mebutamol (0.40 g, 1.71 mmol) in 20 ml of anhydrous acetonitrile was added dropwise. Stir the reaction for 30 min, then add dimethylcarbamoyl chloride (195 μl, 2.06 mmol) dropwise, and react for 2 h. Recover the solvent, add 25ml of water to dilute, extract with ethyl acetate (25ml×2), combine the organic layers, wash with water, anhydrous Na 2 SO 4 dry. After filtration and concentration, 0.50 g of light yellow oil was obtained, with a yield of 96%.
[0038] Dissolve 0.50 g of light yellow oil in 5 ml of ethanol, form a salt with anhydrous HCl-EtOH, recover the solvent, and recrystallize with acetone to obtain light yellow needle crysta...
Embodiment 2
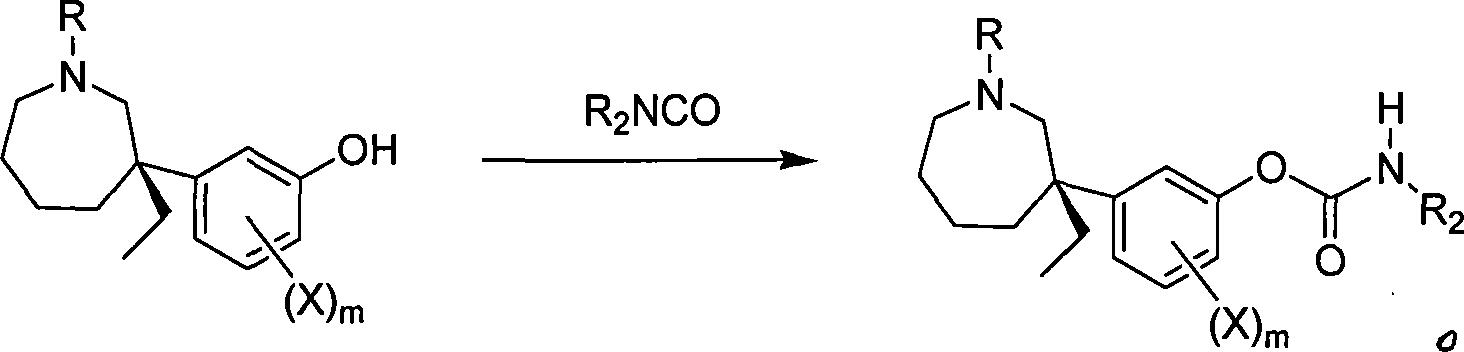
[0039] Embodiment 2 (-)-Mabutaol phenylcarbamate (R 1 = H R 2 =Ph) Preparation
[0040] Sodium hydride (80%, 0.15 g, 5.00 mmol) was added to tetrahydrofuran, cooled, and a tetrahydrofuran solution of (-)-meprotamol (0.40 g, 1.71 mmol) was added dropwise. After stirring for 30 min, phenyl isocyanate (488 μl, 4.48 mmol) was added dropwise and reacted for 4 h. After recovering the solvent, adjust to alkaline with dilute ammonia water, and finally extract with diethyl ether (20ml×2), anhydrous Na 2 SO 4 dry. After filtration and concentration, 0.43 g of off-white solid was obtained, with a yield of 71.7%.
[0041] 0.43 g of off-white solid was dissolved in anhydrous ether, and HCl-anhydrous ether was added dropwise to form a salt, and a solid was precipitated. Filter, wash with a small amount of anhydrous ether, and dry in vacuo. Obtain (-)-mabutamol phenylcarbamate hydrochloride powder 0.39g, yield 82.1%, m.p.122~127 ℃, [α] D = -18.94° (c 0.108, MeOH). 1 HNMR (DMSO-d 6 ...
Embodiment 3
[0042] Embodiment 3 cholinesterase inhibitory activity test method:
[0043] The enzyme inhibitory activity was measured by Ellman colorimetric method. According to the hydrolysis of acetylcholine by acetylcholinesterase, choline and acetic acid were generated, and choline reacted with sulfhydryl chromogen to generate a yellow compound. The quantity reflects the experimental principle of acetylcholinesterase activity, and the cholinesterase activity was determined according to the instructions of the AChE kit provided by Nanjing Jiancheng Biotechnology Co., Ltd. The source of AChE enzyme is 10% homogenate of rat brain tissue (made by adding physiological saline), and the source of BChE enzyme is rat serum. Measure the cholinesterase activity after reacting different concentrations of the drug to be tested with rat brain homogenate supernatant or rat serum for 20 minutes to calculate its IC 50 value.
[0044] Results IC with three measurements 50 Mean value (mean) ± standard...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com